Muscle Rub Ultra
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Muscle Rub Ultra Uses
- Directions
- Muscle Rub Ultra Other information
- Inactive Ingredients
- Questions?
FULL PRESCRIBING INFORMATION
Active Ingredients
Camphor 4%
Menthol 10%
Methyl Salicylate 30%
Muscle Rub Ultra Uses
- simple bachache
- arthritis
- strains
- bruises
- sprains
For external use only.
Do not use
- on wounds or damaged skin
- with a heating pad
- on a child under 12 years of age with arthritis-like conditions
Ask a doctor before use if you have
redness over the affected area
When using this product
- avoid contact with eyes or mucous membranes
- do not bandage tightly
Stop use and ask a doctor if
- condition worsens or symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
- excessive skin irritation occurs
Keep out of reach of children to avoid accidental ingestion.
If swallowed, get medical help or contact a Poison Control Center immediately
Directions
- Use only as directed
- adults and children 12 years of age and older:
- children under 12 years of age: ask a doctor
Muscle Rub Ultra Other information
-
Store at 20 ° to 25 ° C (68 ° to 77 °F)
Inactive Ingredients
carbomer, edetate disodium, glyceryl stearate se, lanolin, polysorbate 80, potassium hydroxide, purified water, stearic acid, trolamine
Questions?
call 1-800-883-0085, weekdays, 9AM -5PM PST
Muscle Rub Ultra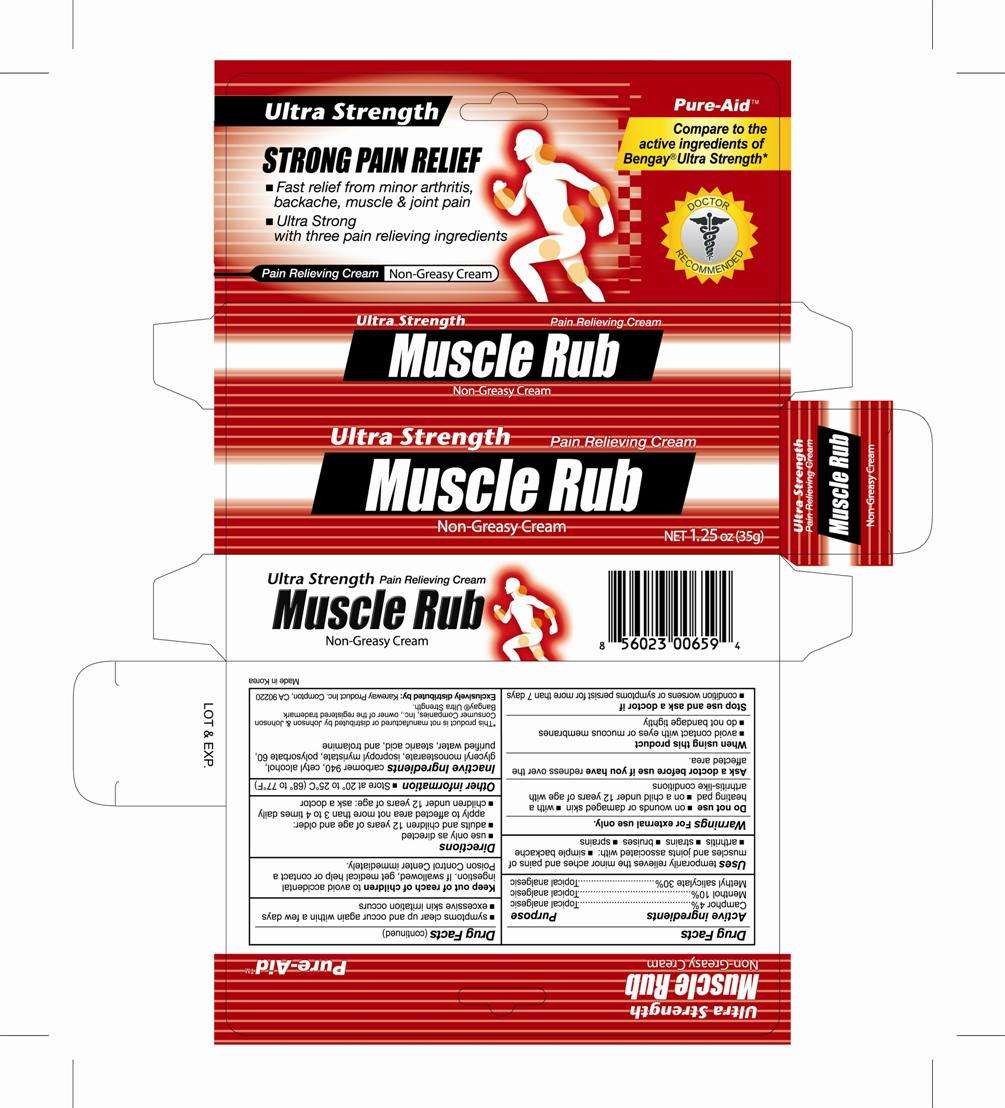
Purpose
Topical analgesic
Topical analgesic
Topical analgesic
Muscle Rub UltraCamphor,Menthol, methyl salicylate CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||