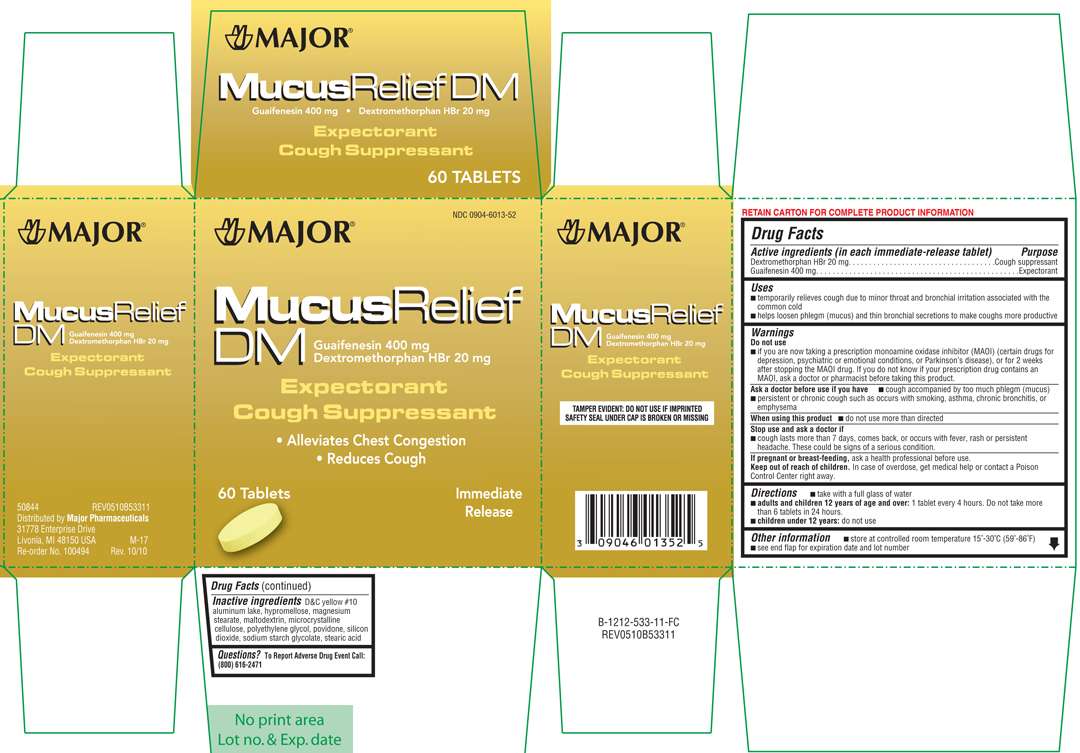
Mucus Relief DM
Major 44-533
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients
- Purpose
- Mucus Relief DM Uses
- Warnings
- Directions
- Mucus Relief DM Other information
- Inactive ingredients
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredients
Dextromethorphan HBr 20 mg
Guaifenesin 400 mg
Purpose
Cough suppressant
Expectorant
Mucus Relief DM Uses
-
temporarily relieves cough due to minor throat and bronchial irritation associated with the common cold
-
helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
Warnings
Do not use
-
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
-
cough accompanied by too much phlegm (mucus)
-
persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
When using this product
-
do not use more than directed
Stop use and ask a doctor if
-
cough lasts more than 7 days, comes back, or occurs with fever, rash or persistent headache. These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
-
take with a full glass of water
-
adults and children 12 years of age and over: 1 tablet every 4 hours. Do not take more than 6 tablets in 24 hours.
-
children under 12 years: do not use
Mucus Relief DM Other information
- store at controlled room temperature 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Inactive ingredients
D&C yellow #10 aluminum lake, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, povidone, silicon dioxide, sodium starch glycolate, stearic acid
Principal Display Panel
Major®
Mucus Relief DM
Guaifenesin 400 mg
Dextromethorphan HBr 20 mg
Expectorant
Cough Suppressant
• Alleviates Chest Congestion
• Reduces Cough
60 Tablets
Immediate Release
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
50844 REV0510B53311
Distributed by Major Pharmaceuticals
31778 Enterprise Drive
Livonia, MI 48150 USA M-17
Re-order No. 100494 Rev. 10/10
Mucus Relief DMDextromethorphan HBr and Guiafenesin TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||