Mucinex Fast-Max
Mucinex® Fast-Max Severe Congestion and Cough
FULL PRESCRIBING INFORMATION: CONTENTS*
- Mucinex Fast-Max Uses
- Warnings
- Directions
- Mucinex Fast-Max Other information
- Inactive ingredients
- Questions?
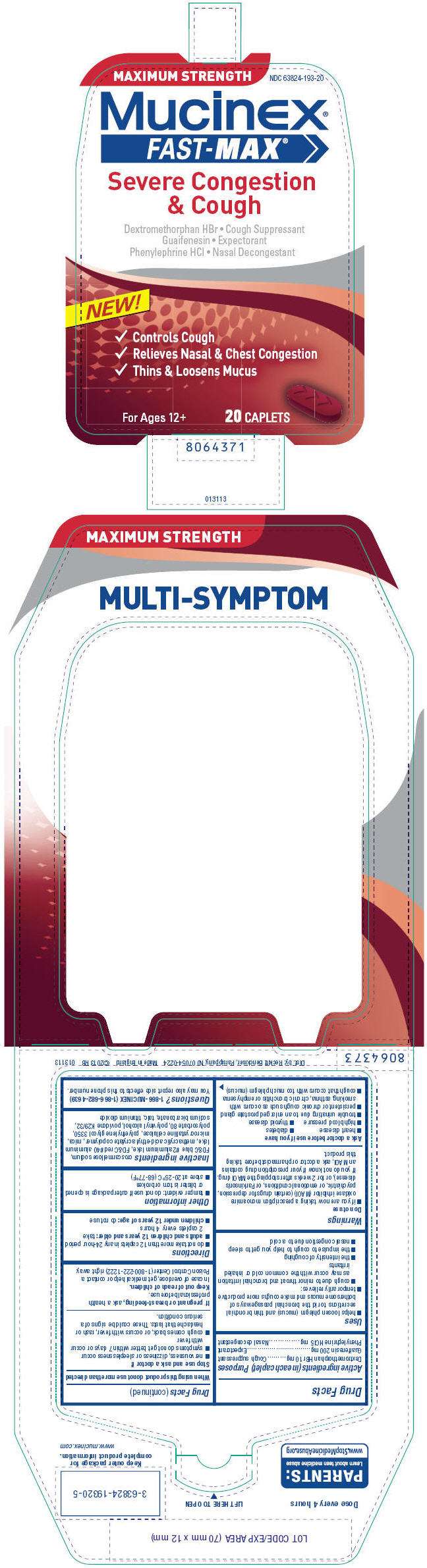
- PRINCIPAL DISPLAY PANEL - 2 x 10 Caplet Blister Pack Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active ingredients (in each caplet) | Purposes |
|---|---|
| Dextromethorphan HBr 10 mg | Cough suppressant |
| Guaifenesin 200 mg | Expectorant |
| Phenylephrine HCl 5 mg | Nasal decongestant |
Mucinex Fast-Max Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves:
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- the intensity of coughing
- the impulse to cough to help you get to sleep
- nasal congestion due to a cold
Warnings
Do not use
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- diabetes
- high blood pressure
- thyroid disease
- trouble urinating due to an enlarged prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis or emphysema
- cough that occurs with too much phlegm (mucus)
When using this product do not use more than directed
Stop use and ask a doctor if
- nervousness, dizziness or sleeplessness occur
- symptoms do not get better within 7 days or occur with fever
- cough comes back, or occurs with fever, rash or headache that lasts. These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- do not take more than 12 caplets in any 24-hour period
- adults and children 12 years and older: take 2 caplets every 4 hours
- children under 12 years of age: do not use
Mucinex Fast-Max Other information
- tamper evident: do not use if outer package is opened or blister is torn or broken
- store at 20-25°C (68-77°F)
Inactive ingredients
croscarmellose sodium, FD&C blue #2 aluminum lake, FD&C red #40 aluminum lake, methacrylic acid-ethyl acrylate copolymer, mica, microcrystalline cellulose, polyethylene glycol 3350, polysorbate 80, polyvinyl alcohol, povidone K29/32, sodium bicarbonate, talc, titanium dioxide
Questions?
1-866-MUCINEX (1-866-682-4639)
You may also report side effects to this phone number.
Dist. by: Reckitt Benckiser, Parsippany, NJ 07054-0224
Made in England
PRINCIPAL DISPLAY PANEL - 2 x 10 Caplet Blister Pack Carton
MAXIMUM STRENGTH
NDC 63824-193-20
Mucinex®
FAST-MAX
®
Severe Congestion
& Cough
Dextromethorphan HBr • Cough Suppressant
Guaifenesin • Expectorant
Phenylephrine HCl • Nasal Decongestant
NEW!
✓ Controls Cough
✓ Relieves Nasal & Chest Congestion
✓ Thins & Loosens Mucus
For Ages 12+
20 CAPLETS
Mucinex Fast-MaxGuaifenesin, Phenylephrine Hydrochloride, and Dextromethorphan hydrobromide TABLET, COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||