Motion-Time
DRUG FACTS
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredient (in each tablet)Purpose
PurposeUses
UsesWarnings
Do not take unless directed by a doctor if you have
- trouble urinating due to an enlarged prostate gland
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
When using this product
- do not exceed recommended dosage
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Keep out of reach of children.
Directions
- dosage should be taken one hour before travel starts
- adults and children 12 years of age and over: take 1 to 2 tablets once daily or as directed by a doctor
- store at 25°C (77°F) excursions permitted between 15°-30°C (59°-86°F) in a dry place
- use by expiration date on package
Meclizine HCL Chewable Tablets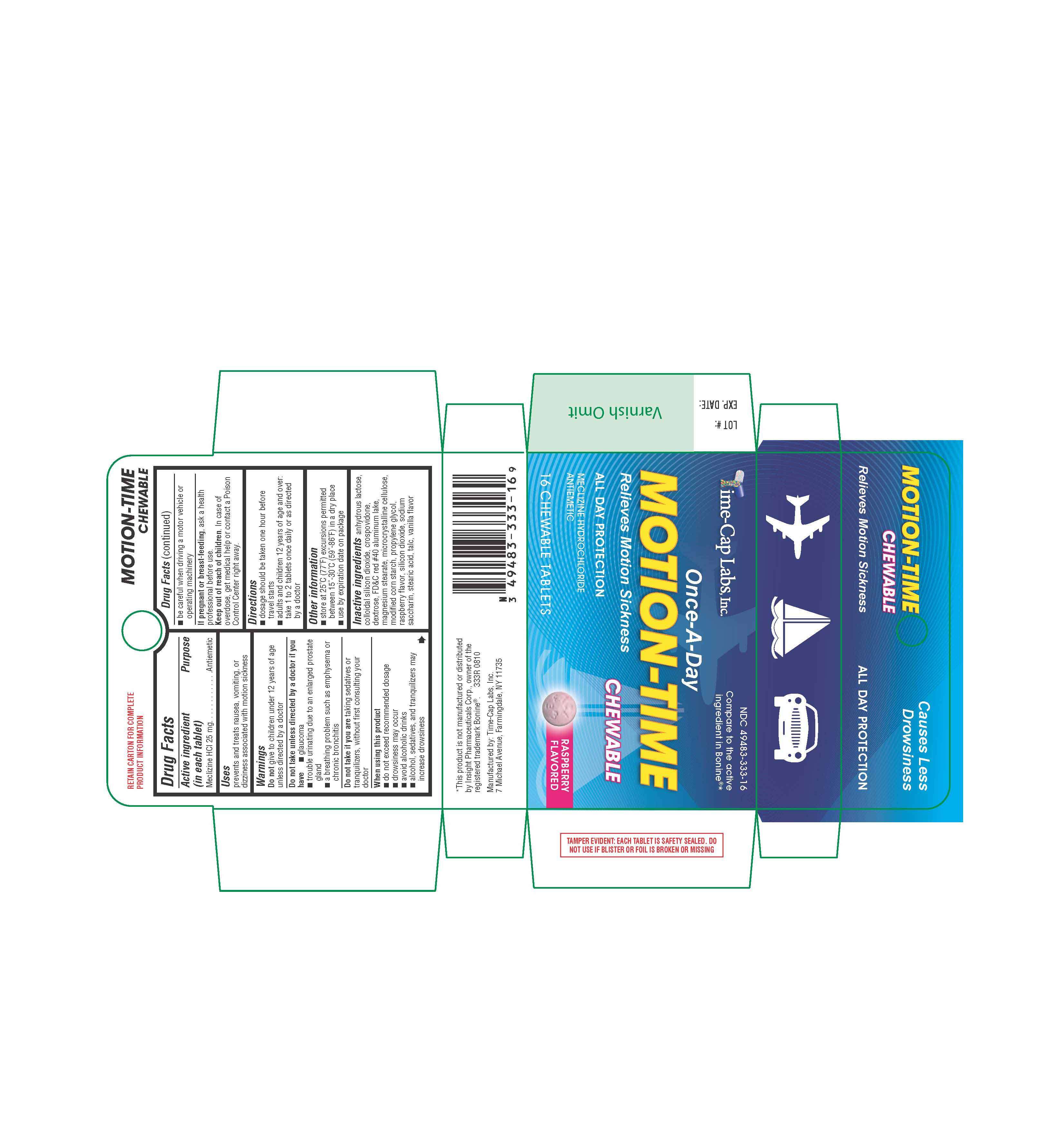
Motion-TimeMeclizine HCl TABLET, CHEWABLE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!