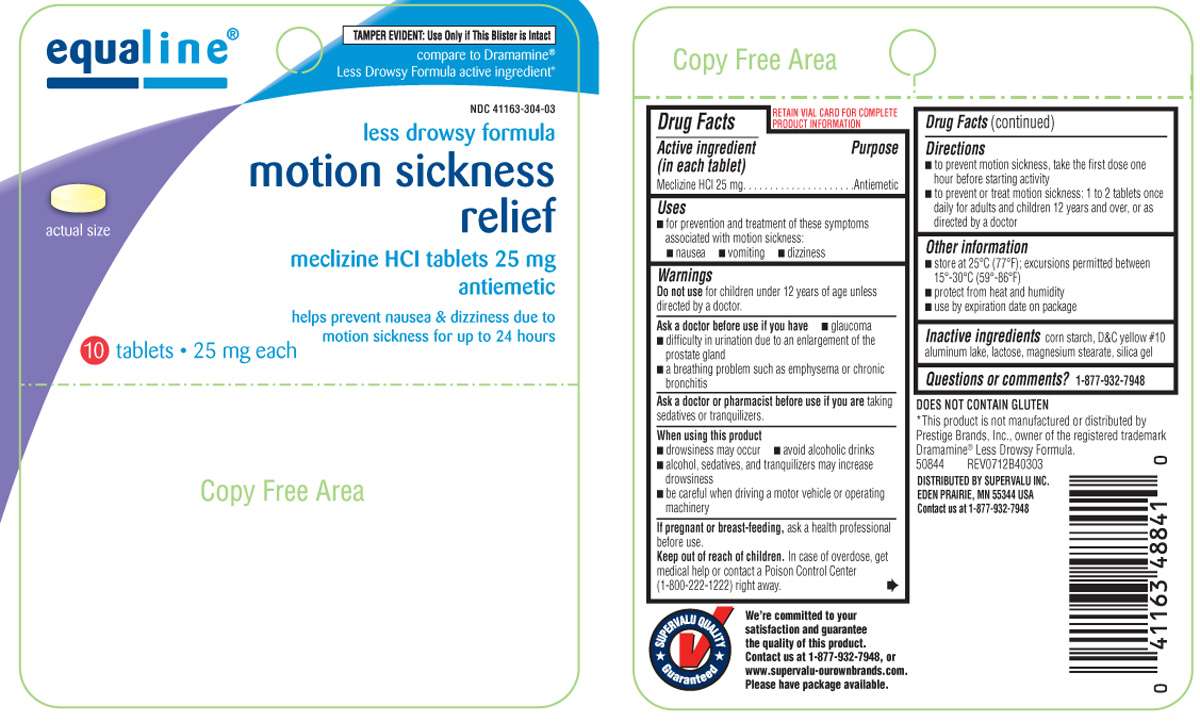
Motion Sickness Relief
Equaline 44-403
FULL PRESCRIBING INFORMATION
Meclizine HCl 25 mg
Antiemetic
- for prevention and treatment of these symptoms associated with motion sickness
- nausea
- vomiting
- dizziness
for children under 12 years of age unless directed by a doctor.
- glaucoma
- difficulty in urination due to an enlargement of the prostate gland
- a breathing problem such as emphysema or chronic bronchitis
taking sedatives or tranquilizers.
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
- to prevent motion sickness, take the first dose one hour before starting activity
- to prevent or treat motion sickness: 1 to 2 tablets once daily for adults and children 12 years and over, or as directed by a doctor
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- protect from heat and humidity
- use by expiration date on package
corn starch, D&C yellow #10 aluminum lake, lactose, magnesium stearate, silica gel
1-877-932-7948
equaline®
TAMPER EVIDENT: Use Only if This Blister is Intact
compare to Dramamine® Less Drowsy Formula active ingredient*
NDC 41163-304-03
less drowsy formula
motion sickness relief
meclizine HCl tablets 25 mg
antiemetic
helps prevent nausea & dizziness due to motion sickness for up to 24 hours
10 tablets • 25 mg each
DOES NOT CONTAIN GLUTEN
*This product is not manufactured or distributed by Prestige Brands, Inc., owner of the registered trademark Dramamine® Less Drowsy Formula.
50844 REV0712B40303
DISTRIBUTED BY SUPERVALU INC.
EDEN PRAIRIE, MN 55344 USA
Contact us at 1-877-932-7948
Motion Sickness ReliefMeclizine HCl TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||