Mirtazapine
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- MIRTAZAPINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- MIRTAZAPINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- MIRTAZAPINE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
MIRTAZAPINE DESCRIPTION
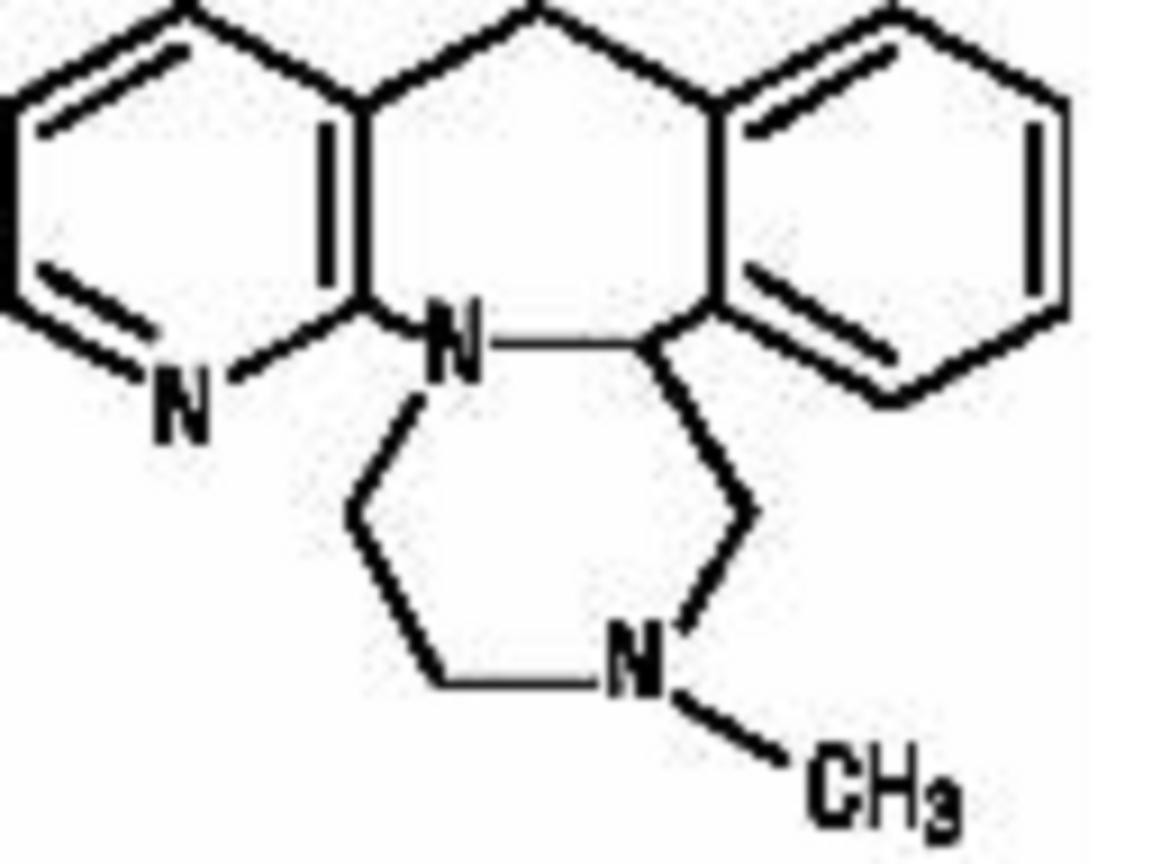
CLINICAL PHARMACOLOGY
PharmacodynamicsPharmacokinetics
Special Populations
Geriatrics
Pediatrics
Gender
Race
Renal Insufficiency
Hepatic Insufficiency
Clinical Trials Showing Effectiveness
INDICATIONS & USAGE
MIRTAZAPINE CONTRAINDICATIONS
HypersensitivityMonoamine Oxidase Inhibitors
WARNINGS
Clinical Worsening and Suicide RiskAll patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers.Such monitoring should include daily observation by families and caregivers.
Screening Patients for Bipolar Disorder
Agranulocytosis
In premarketing clinical trials, 2 (1 with Sj's Syndrome) out of 2796 patients treated with mirtazapine tablets developed agranulocytosis [absolute neutrophil count (ANC) < 500/mm3 with associated signs and symptoms, e.g., fever, infection, etc.] and a third patient developed severe neutropenia [ANC < 500/mm3 without any associated symptoms]. For these 3 patients, onset of severe neutropenia was detected on days 61, 9, and 14 of treatment, respectively. All 3 patients recovered after mirtazapine was stopped. These 3 cases yield a crude incidence of severe neutropenia (with or without associated infection) of approximately 1.1 per thousand patients exposed, with a very wide 95% confidence interval, i.e., 2.2 cases per 10,000 to 3.1 cases per 1000. If a patient develops a sore throat, fever, stomatitis, or other signs of infection, along with a low WBC count, treatment with mirtazapine should be discontinued and the patient should be closely monitored.
MAO Inhibitors
In patients receiving other drugs for major depressive disorder in combination with a monoamine oxidase inhibitor (MAOI) and in patients who have recently discontinued a drug for major depressive disorder and then are started on an MAOI, there have been reports of serious and sometimes fatal reactions, including nausea, vomiting, flushing, dizziness, tremor, myoclonus, rigidity, diaphoresis, hyperthermia, autonomic instability with rapid fluctuations of vital signs, seizures, and mental status changes ranging from agitation to coma. Although there are no human data pertinent to such an interaction with mirtazapine tablets, it is recommended that mirtazapine not be used in combination with an MAOI, or within 14 days of initiating or discontinuing therapy with an MAOI.
Serotonin Syndrome
PRECAUTIONS
GeneralDiscontinuation Symptoms
Akathisia/Psychomotor Restlessness
Hyponatremia
Somnolence
Dizziness
Increased Appetite/Weight Gain
Cholesterol/Triglycerides
Transaminase Elevations
Activation of Mania/Hypomania
Seizure
Use in Patients With Concomitant Illness
INFORMATION FOR PATIENTS
Clinical Worsening and Suicide Risk
Agranulocytosis
Interference With Cognitive and Motor Performance
Completing Course of Therapy
Concomitant Medication
Alcohol
Pregnancy
Nursing
LABORATORY TESTS
DRUG INTERACTIONS
Monoamine Oxidase Inhibitors
Serotonergic Drugs
Drugs Affecting Hepatic Metabolism
Drugs That are Metabolized by and/or Inhibit Cytochrome P450 Enzymes
Other Drug-Drug Interactions
Alcohol
Diazepam
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CarcinogenesisMutagenesis
Impairment of Fertility
PREGNANCY
Teratogenic EffectsPregnancy category C
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
MIRTAZAPINE ADVERSE REACTIONS
Associated With Discontinuation of TreatmentCommonly Observed Adverse Events in U.S. Controlled Clinical Trials
Adverse Events Occurring at an Incidence of 1% or More Among Mirtazapine-Treated Patients
*
*
ECG Changes
Other Adverse Events Observed During the Premarketing Evaluation of Mirtazapine
Other Adverse Events Observed During Postmarketing Evaluation of Mirtazapine
DRUG ABUSE AND DEPENDENCE
Controlled Substance ClassPhysical and Psychological Dependence
OVERDOSAGE
Human ExperienceOverdose Management
DOSAGE & ADMINISTRATION
Initial TreatmentElderly and Patients With Renal or Hepatic Impairment
Maintenance/Extended Treatment
Switching Patients To or From a Monoamine Oxidase Inhibitor
Discontinuation of Mirtazapine Treatment
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
-
● all risks and benefits of treatment with antidepressant medicines
-
● all treatment choices for depression or other serious mental illness
-
● Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
-
● Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
-
● Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
-
● thoughts about suicide or dying
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling very agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● other unusual changes in behavior or mood
-
● Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
-
● Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
-
● Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
-
● Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
-
● Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information.
-
● are allergic to mirtazapine or any of the ingredients in mirtazapine tablets USP. See the end of the Medication Guide for a complete list of ingredients in mirtazapine tablets USP.
-
● currently take or have taken within the last 14 days, any medicine known as Monoamine Oxidase Inhibitors (MAOI). Taking an MAOI with certain other medicines, with similar actions to mirtazapine tablets USP, can cause serious or even life-threatening side effects.
-
● have or had liver problems
-
● have or had kidney problems
-
● have or had manic episodes
-
● have had a seizure (convulsion)
-
● have any heart problems
-
● tend to get dizzy or faint
-
● are pregnant or planning to become pregnant. It is not known if mirtazapine tablets USP will harm your unborn baby.
-
● are breastfeeding. It is not known if mirtazapine passes into your milk or if it can harm your unborn baby.
-
● Take mirtazapine tablets USP exactly as prescribed by your doctor.
-
● Take mirtazapine tablets USP at the same time each day, preferably in the evening at bedtime.
-
● Swallow mirtazapine tablets USP as directed.
-
● It is common for antidepressant medicines such as mirtazapine tablets USP to take up to a few weeks before you start to feel better. Do not stop taking mirtazapine tablets USP if you do not feel results right away.
-
● Do not stop taking or change the dose of mirtazapine tablets USP without talking to your doctor, even if you feel better.
-
● If you miss a dose of mirtazapine tablets USP, do not take another dose to make up for the dose you forgot. Wait and take your tablet at the next regular time.
-
● If you take too much mirtazapine tablets USP, call your doctor or poison control center or go to the emergency room right away.
-
● Mirtazapine tablets USP can cause drowsiness, which may affect your ability to make decisions, think clearly or react quickly. You should not drive, operate heavy machinery or do other dangerous activities until you know how mirtazapine tablets USP affect you.
-
● Avoid drinking alcohol or taking diazepam (a medicine used for anxiety, insomnia and seizures, for example) or similar medicines, while taking mirtazapine tablets USP. If you are unsure about whether a certain medication can be taken together with mirtazapine tablets USP, please discuss this with your doctor.
-
● See the beginning of this Medication Guide - Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or Actions
-
● Serotonin Syndrome: This is a condition that can be life threatening. Call your doctor right away if you become severely ill and have some or all of these symptoms:
-
● stiffness
-
● muscle spasm
-
● confusion
-
● irritability
-
● agitation
-
● increased body temperature
-
● fast heart rate
-
● increased blood pressure
-
● Decreased White Blood Cells called neutrophils, which are needed to fight infections. Tell your doctor if you have any indication of infection such as fever, chills, sore throat, or mouth or nose sores, especially symptoms which are flu-like.
-
● Increased cholesterol and triglyceride levels in your blood
-
● Symptoms when stopping mirtazapine tablets USP (discontinuation symptoms). Side effects may occur when stopping mirtazapine tablets USP (discontinuation symptoms), especially when therapy is stopped suddenly. Your healthcare provider may want to decrease your dose slowly to help avoid side effects. Some of these side effects may include:
-
● dizziness
-
● abnormal dreams
-
● agitation
-
● anxiety
-
● fatigue
-
● confusion
-
● headache
-
● shaking
-
● tingling sensation
-
● nausea
-
● vomiting
-
● sweating
-
● Mania
-
● Seizures
-
● Low sodium levels in your blood: Call your doctor right away if you become severely ill and have some or all of these symptoms:
-
● headache
-
● feel weak
-
● confusion
-
● problems concentrating
-
● memory problems
-
● feel unsteady
-
● Store at 20to 25(68to 77
-
● Protect from light and moisture.
-
● Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
-
● Do not use mirtazapine tablets USP for a condition for which they were not prescribed. Do not give mirtazapine tablets USP to other people, even if they have the same condition as you have. They may harm them.
-
● This Medication Guide summarizes the most important information about mirtazapine tablets USP. If you have any concerns or would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about mirtazapine tablets USP that is written for health professionals.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

MirtazapineMirtazapine TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!