Mirtazapine
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- MIRTAZAPINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS
- USE IN SPECIFIC POPULATIONS
- INDICATIONS & USAGE
- MIRTAZAPINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- MIRTAZAPINE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Suicidality and Antidepressant DrugsAntidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of mirtazapine tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase int eh risk of suicidality with antidpressants compared to placebo in adults beyone age 24; there was a reduction in rish with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated wiht increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Mirtazapine is not approved for use in pediatric patients. (See Warnings: Clinical Worsening and Suicide Risk,Precautions: Information for Patients, andPrecautions: Pediatric Use)
MIRTAZAPINE DESCRIPTION
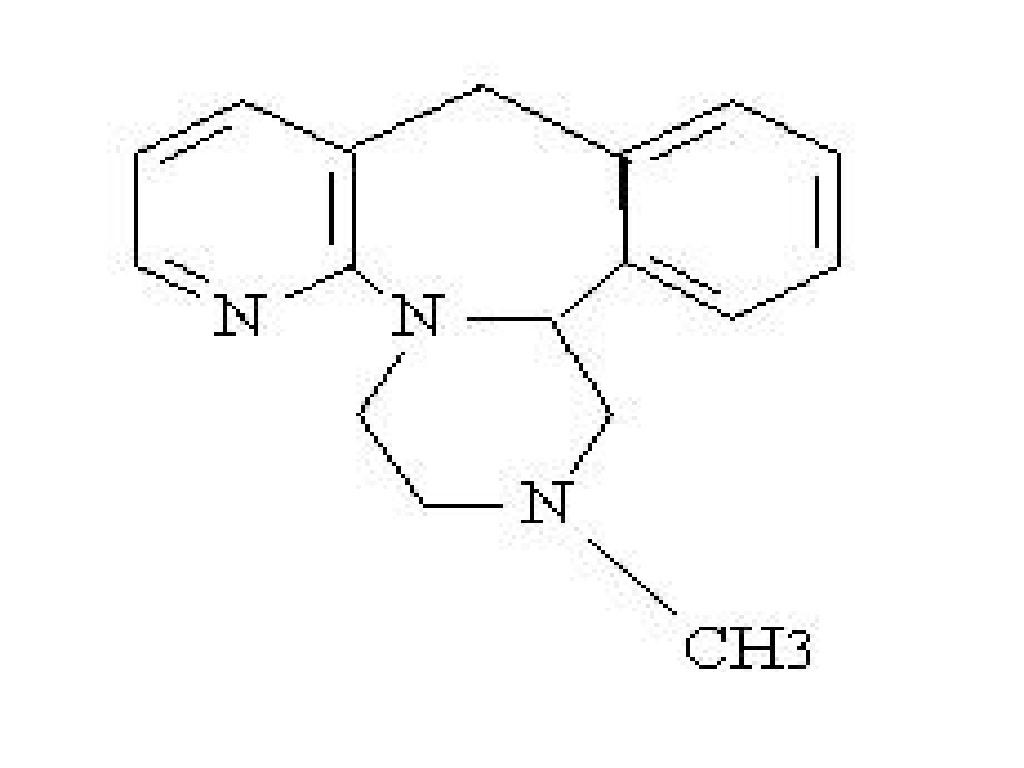
CLINICAL PHARMACOLOGY
PHARMACODYNAMICS
PHARMACOKINETICS
USE IN SPECIFIC POPULATIONS
PRECAUTIONSDOSAGE AND ADMINISTRATION
PRECAUTIONS
Pharmacokinetics
PRECAUTIONSDOSAGE AND ADMINISTRATION
PRECAUTIONSDOSAGE AND ADMINISTRATION
Clinical Trials Showing Effectiveness
INDICATIONS & USAGE
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
MIRTAZAPINE CONTRAINDICATIONS
HypersensitivityMonoamine Oxidase Inhibitors
WARNINGSPRECAUTIONS: Drug InteractionsDOSAGE AND ADMINISTRATION
WARNINGS
Clinical Worsening and Suicide RiskTable 1
Agranulocytosis
MAO Inhibitors
Serotonin Syndrome
CONTRAINDICATIONSPRECAUTIONS: Drug Interactions
PRECAUTIONS
GeneralInformation for Patients
PRECAUTIONS: Pediatric Use
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
INFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
CLINICAL PHARMACOLOGYCONTRAINDICATIONSWARNINGSDOSAGE AND ADMINISTRATION
CONTRAINDICATIONSWARNINGS
Other Drug-Drug Interactions
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
BOX WARNING and WARNINGSClinical Worsening and Suicide RiskPRECAUTIONS-Increased Appetite/Weight Gain
GERIATRIC USE
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATIONMIRTAZAPINE ADVERSE REACTIONS
Associated with Discontinuation of TreatmentCommonly Observed Adverse Events in US Controlled Clinical Trials
Adverse Events Occurring at an Incidence of 1% or More Among Mirtazapine-Treated Patients
Body System Adverse Clinical ExperienceMirtazapine (n=453)Placebo (n=361)
ECG Changes
Other Adverse Events Observed During the Premarketing Evaluation of Mirtazapine
WARNINGSPRECAUTIONS
Other Adverse Events Observed During Postmarketing Evaluation of Mirtazapine
DRUG ABUSE AND DEPENDENCE
Controlled Substance ClassPhysical and Psychological Dependence
OVERDOSAGE
Human ExperienceOverdose Management
DOSAGE & ADMINISTRATION
Initial TreatmentElderly and Patients with Renal or Hepatic Impairment
PRECAUTIONSCLINICAL PHARMACOLOGY
Maintenance/Extended Treatment
CLINICAL PHARMACOLOGY
Switching Patients To or From a Monoamine Oxidase Inhibitor
Discontinuation of Mirtazapine Tablets treatment
PRECAUTIONSADVERSE REACTIONS
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
Medication Guide-
● all risks and benefits of treatment with antidepressant medicines
-
● all treatment choices for depression or other serious mental illness
-
● Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
-
● Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
-
● Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
-
● thoughts about suicide or dying
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling very agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● other unusual changes in behavior or mood
-
● Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
-
● Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
-
● Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
-
● Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
-
● Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information.
-
● are allergic to mirtazapine or any of the ingredients in mirtazapine tablets. See the end of the Medication Guide for a complete list of ingredients in mirtazapine tablets.
-
● currently take or have taken within the last 14 days, any medicine known as Monoamine Oxidase inhibitors (MAOI). Taking an MAOI with certain other medicines, with similar actions to mirtazapine tablets, can cause serious or even life-threatening side effects.
-
● have or had liver problems
-
● have or had kidney problems
-
● have or had manic episodes
-
● have had a seizure (convulsion)
-
● have any heart problems
-
● tend to get dizzy or faint
-
● are pregnant or planning to become pregnant. It is not known if mirtazapine will harm your unborn baby.
-
● are breastfeeding. It is not known if mirtazapine passes into your milk or if it can harm your unborn baby.
-
● Take mirtazapine tablets exactly as prescribed by your doctor.
-
● Take mirtazapine tablets at the same time each day, preferably in the evening at bedtime.
-
● Swallow mirtazapine tablets as directed.
-
● It is common for antidepressant medicines such as mirtazapine tablets to take up to a few weeks before you start to feel better. Do not stop taking mirtazapine tablets if you do not feel results right away.
-
● Do not stop taking or change the dose of mirtazapine tablets without talking to your doctor, even if you feel better.
-
● If you miss a dose of mirtazapine tablets, do not take another dose to make up for the dose you forgot. Wait and take your tablet at the next regular time.
-
● If you take too much mirtazapine tablets, call your doctor or poison control center or go to the emergency room right away.
-
● Mirtazapine tablets can cause drowsiness, which may affect your ability to make decisions, think clearly or react quickly. You should not drive, operate heavy machinery or do other dangerous activities until you know how mirtazapine tablets affect you.
-
● Avoid drinking alcohol or taking diazepam (a medicine used for anxiety, insomnia and seizures, for example) or similar medicines, while taking mirtazapine tablets. If you are unsure about whether a certain medication can be taken together with mirtazapine tablets, please discuss this with your doctor.
-
● See the beginning of this Medication Guide - Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or Actions
-
● Serotonin Syndrome: This is a condition that can be life threatening. Call your doctor right away if you become severely ill and have some or all of these symptoms:
-
● stiffness
-
● muscle spasm
-
● confusion
-
● irritability
-
● agitation
-
● increased body temperature
-
● fast heart rate
-
● increased blood pressure
-
● Decreased White Blood Cells called neutrophils, which are needed to fight infections. Tell your doctor if you have any indication of infection such as fever, chills, sore throat, or mouth or nose sores, especially symptoms which are flu-like.
-
● Increased cholesterol and triglyceride levels in your blood
-
● Symptoms when stopping mirtazapine (discontinuation symptoms). Side effects may occur when stopping mirtazapine (discontinuation symptoms), especially when therapy is stopped suddenly. Your healthcare provider may want to decrease your dose slowly to help avoid side effects. Some of these side effects may include:
-
● dizziness
-
● abnormal dreams
-
● agitation
-
● anxiety
-
● fatigue
-
● confusion
-
● headache
-
● shaking
-
● tingling sensation
-
● nausea
-
● vomiting
-
● sweating
-
● Mania
-
● Seizures
-
● Low sodium levels in your blood: Call your doctor right away if you become severely ill and have some or all of these symptoms:
-
● headache
-
● feel weak
-
● confusion
-
● problems concentrating
-
● memory problems
-
● feel unsteady
-
● Store at 25(77to 30(59to 86[see USP Controlled Room Temperature].
-
● Protect from light and moisture.
-
● Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
-
● Do not use mirtazapine tablets for a condition for which it was not prescribed. Do not give mirtazapine tablets to other people, even if they have the same condition as you have. They may harm them.
-
● This Medication guide summarizes the most important information about mirtazapine tablets. If you have any concerns or would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about mirtazapine tablets that is written for health professionals.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

MirtazapineMirtazapine TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!