Minocycline Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- MINOCYCLINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- MICROBIOLOGY
- INDICATIONS & USAGE
- MINOCYCLINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- MINOCYCLINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- REFERENCES
- SPL PATIENT PACKAGE INSERT
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
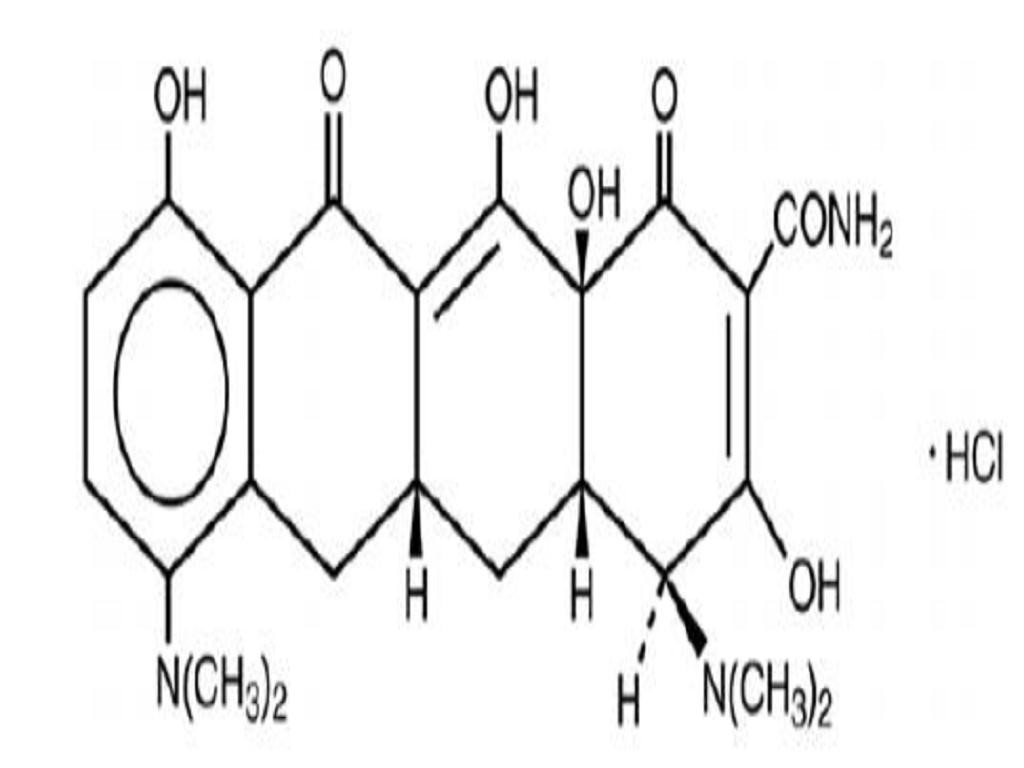
MINOCYCLINE HYDROCHLORIDE DESCRIPTION
DESCRIPTION
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGYMICROBIOLOGY
MicrobiologyINDICATIONS AND USAGE
1
1
1
1
1
1
1
1
1
1
Susceptibility Tests
Dilution techniques
MIC (mcg/mL)Interpretation23
MIC (mcg/mL)Interpretation4
MIC (mcg/mL)Interpretation
MicroorganismMIC Range (mcg/mL)
2
3
4
Diffusion techniques
Zone Diameter (mm)Interpretation5
Zone Diameter (mm)Interpretation6
Zone Diameter (mm)Interpretation7
Zone Diameter (mm)Interpretation8
Zone Diameter (mm)Interpretation
MicroorganismZone Diameter Range (mm)TetracyclineMinocycline
5
6
7
8
INDICATIONS & USAGE
INDICATIONS AND USAGEMINOCYCLINE HYDROCHLORIDE CONTRAINDICATIONS
CONTRAINDICATIONSWARNINGS
DOSAGE AND ADMINISTRATION
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
Information For PatientsWARNINGS
Drug Interactions
LABORATORY TESTS
Laboratory TestsDRUG INTERACTIONS
Drug InteractionsPRECAUTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
Drug/Laboratory Test InteractionsCARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category D
WARNINGS
Nonteratogenic Effects
WARNINGS
LABOR & DELIVERY
Labor and DeliveryNURSING MOTHERS
Nursing MothersWARNINGS
PEDIATRIC USE
Pediatric UseWARNINGS
GERIATRIC USE
Geriatric UseWARNINGSDOSAGE AND ADMINISTRATION
MINOCYCLINE HYDROCHLORIDE ADVERSE REACTIONS
DOSAGE AND ADMINISTRATION
PRECAUTIONS
WARNINGS
WARNINGS
PRECAUTIONS- General
WARNINGS
OVERDOSAGE
OVERDOSAGEDOSAGE & ADMINISTRATION
CLINICAL PHARMACOLOGY
For Pediatric Patients Above 8 Years Of Age
Adults
WARNINGS
HOW SUPPLIED
STORAGE AND HANDLING
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
ANIMAL PHARMACOLOGY AND TOXICOLOGYREFERENCES
SPL PATIENT PACKAGE INSERT
PATIENT INFORMATIONMINOCYCLINE HYDROCHLORIDE CAPSULES
50 mg, 75 mg, and 100 mg
ingredients
-
● have liver or kidney problems
-
● are pregnant or planning to become pregnant. Minocycline hydrochloride capsules may harm your unborn baby. Stop taking minocycline hydrochloride capsules and call your doctor if you become pregnant while taking it.
-
● are breast feeding. Minocycline hydrochloride passes into your milk and may harm your baby. You should decide whether to use minocycline hydrochloride capsules or breastfeed, but not both.
-
● birth control pills. Minocycline hydrochloride capsules may make your birth control pills less effective
-
● a blood thinner medicine. The dose of your blood thinner may have to be lowered.
-
● a penicillin antibiotic medicine. Minocycline hydrochloride capsules and penicillins should not be used together.
-
● Migraine medicines called ergot alkaloids
-
● An acne medicine called isotretinoin
-
● Antacids that contain aluminum, calcium, or magnesium, or iron-containing products.
-
● Take minocycline hydrochloride capsules exactly as your doctor tells you to take them. Skipping doses or not taking all your minocycline hydrochloride capsules may:
-
● Take minocycline hydrochloride capsules with a full glass of liquid. Taking minocycline hydrochloride capsules with enough liquid may lower your chance of getting irritation or ulcers in your esophagus. Your esophagus is the tube that connects your mouth to your stomach.
-
● Minocycline hydrochloride capsules may be taken with or without food. If you forget to take minocycline hydrochloride capsules, take it as soon as you remember.
-
● If you take too much minocycline hydrochloride capsules, call you doctor or poison control center right away.
-
● watery diarrhea
-
● bloody stools
-
● stomach cramps
-
● unusual headaches
-
● blurred vision
-
● fever
-
● rash
-
● joint pain
-
● feeling very tired
-
● central nervous system effects. Symptoms include light-headedness, dizziness, and a spinning feeling (vertigo). You should not drive or operate machines if you have these symptoms.
-
● sun sensitivity (photosensitivity). You may get a worse sunburn with minocycline hydrochloride capsules. Avoid sun exposure and the use of sunlamps or tanning beds. Protect your skin while out in the sunlight. Stop minocycline hydrochloride capsules and call your doctor if your skin turns red.
-
● Store minocycline hydrochloride capsules at room temperature and away from excess heat and moisture.
-
● Throw away any minocycline hydrochloride capsules that are outdated or no longer needed.
-
● Keep minocycline hydrochloride capsules and all medicines out of the reach of children.
INACTIVE INGREDIENT
INACTIVE INGREDIENTS:LACTOSE MONOHYDRATE
STARCH, CORN
MAGNESIUM STEARATE
TITANIUM DIOXIDE
GELATIN
FERROSOFERRIC OXIDE
FD&C BLUE NO. 1
FERRIC OXIDE YELLOW
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Minocycline HydrochlorideMinocycline Hydrochloride CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!