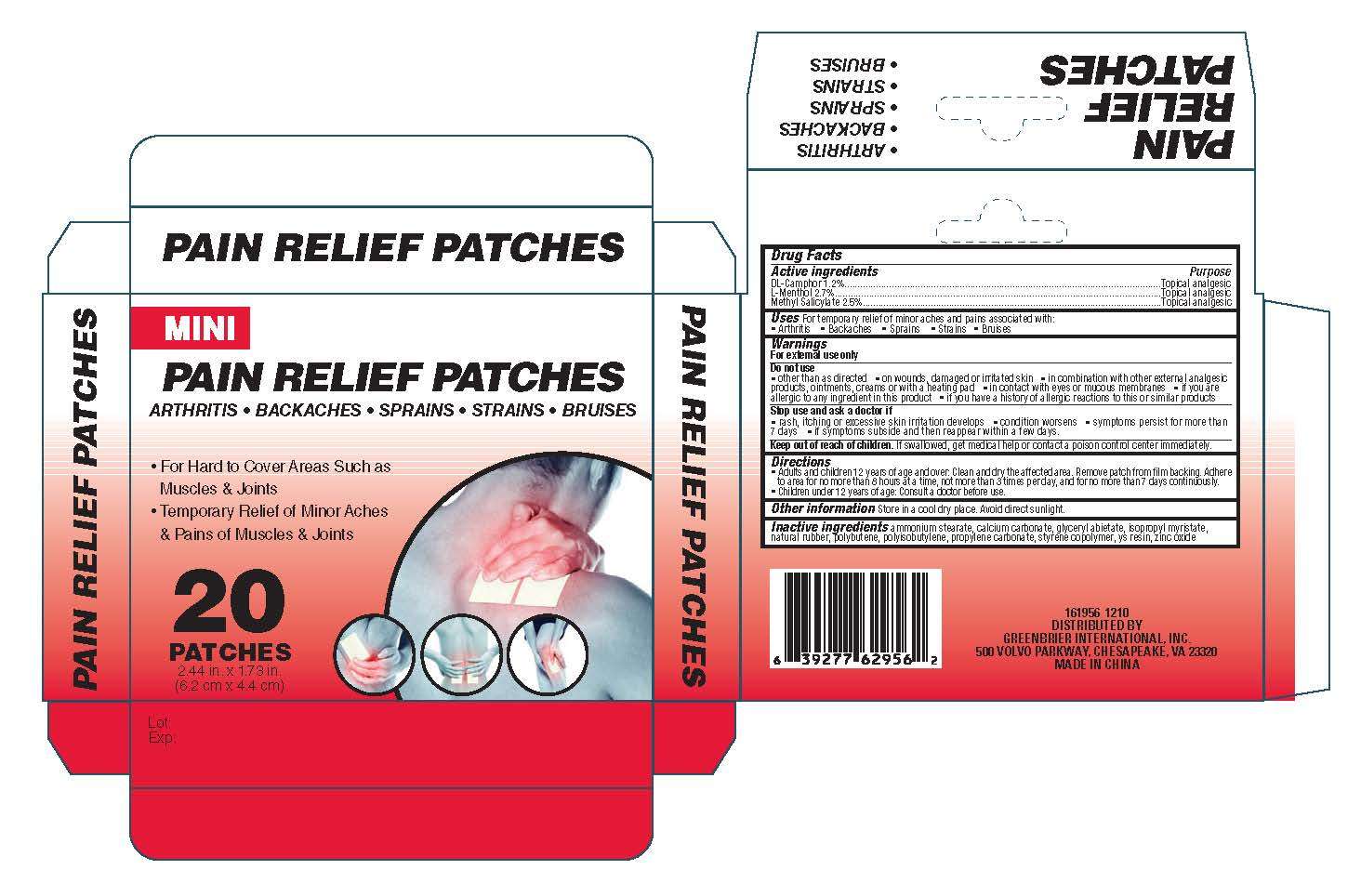
Mini Pain Relief
Shanghai Aquagel Bio-Plymer Co., Ltd
Drug Facts
FULL PRESCRIBING INFORMATION
Inactive ingredients Ammonium Stearate, Calcium Carbonate, Glyceryl Abietate, Isopropyl Myristate, Natural
Rubber,Polybutene, Polyisobutylene, Propylene Carbonate, Styrene Copolymer, YS Resin, Zinc Oxide
Active ingredient
Active ingredients
DL-Camphor 1.2%
L-Menthol 2,.%
Methyl Salicylate 2.5%
Purpose
Purpose
Topical Analgesic
Keep out of reach of children. If swallowed, get medical help or contact a poison control center immediately
Uses
Do not use
other than as directed on wounds, damaged or irritated skin in combination with other
external analgesic products, ointments, creams or with a heating pad. in contact with eyes or mucous
membranes. if you are allergic to any ingredient in this product or if you have a history of allergic reactions to this
or similar products
Stop Use and ask a
doctor if
rash, itching or excessive skin irritation develops
condition worsens
symptoms persist for more
than 7 days
if symptoms
subside and then reappear within a few days.
Warnings
For External Use Only
Directions
Adults and children 12 years of age and over: Clean and dry the affected area. Remove
patch from film backing. Adhere to area for no more than 8 hours at a time, not more than 3 times per day, and for no more
than 7 days continuously. Children under 12 years of age: Consult a doctor before use.
Uses
For temporary relief of minor aches and pains associated with:
Arthritis
Backaches
Sprains
Strains
Bruises
Mini Pain ReliefDL-Camphor L-Menthol Methyl Salicylate PATCH
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||