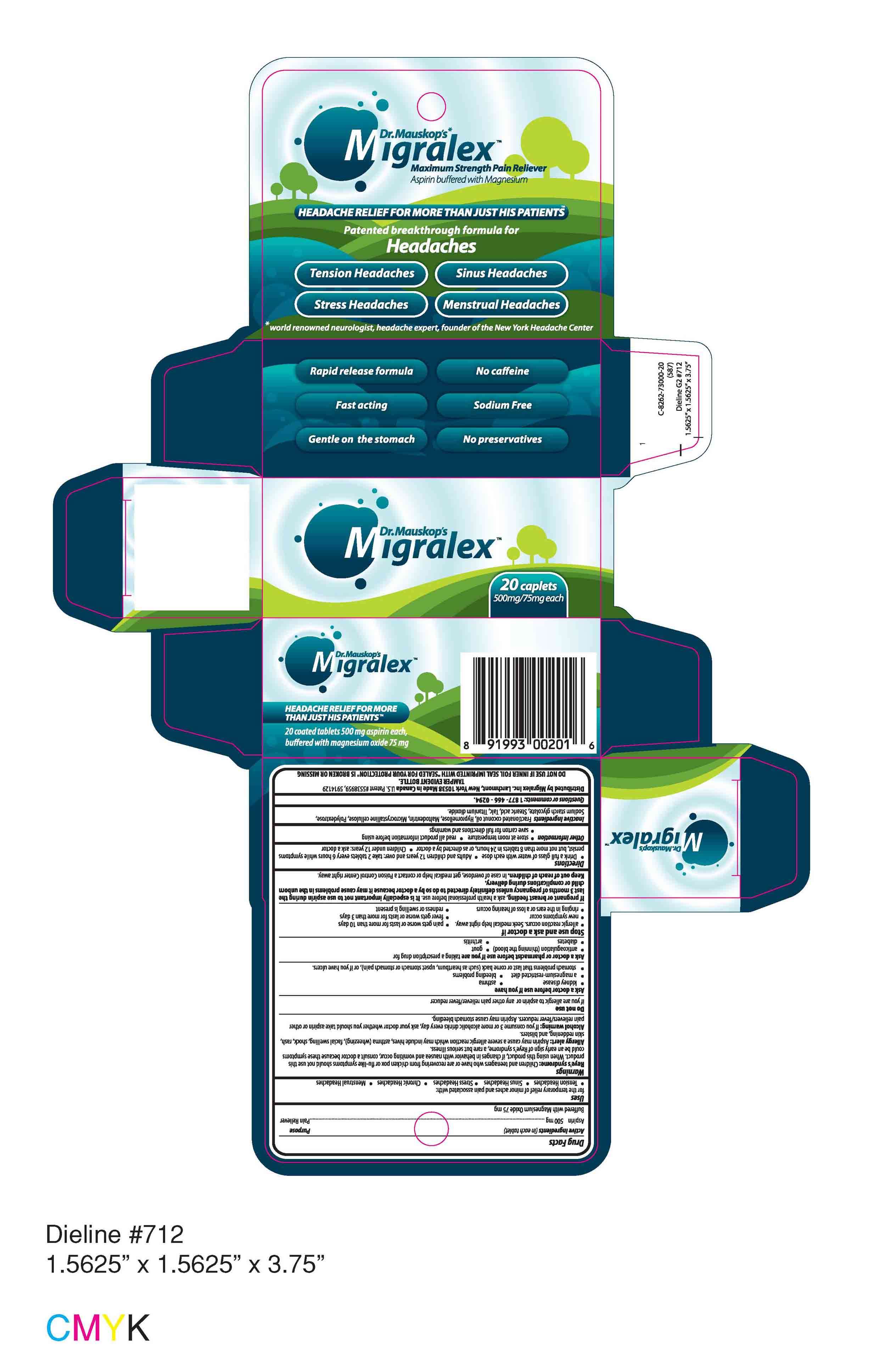
Migralex
Migralex Labeling
FULL PRESCRIBING INFORMATION
Active ingredient
Active IngredientsPurpose
Uses- Tension Headaches
- Sinus Headaches
- Stress Headaches
- Chronic Headaches
- Menstrual Headaches
Reye's syndrome
Allergy alert
Alcohol warning
Do not use
Ask a doctor before use if you have
- kidney disease
- asthma
- a magnesium-restricted diet
- bleeding problems
- stomach problems that last or come back (such as heartburn, upset stomach or stomach pain), or if you have ulcers.
- anticoagulation (thinning the blood)
- gout
- diabetes
- arthritis
- allergic reaction occurs. Seek medical help right away.
- pain gets worse or lasts for more than 10 days
- new symptoms occur
- fever gets worse or lasts for more than 3 days
- ringing in the ears or a loss of hearing occurs
- redness or swelling is present
Directions
- Drink a full glass of water with each dose
- Adults and children 12 years and over: take 2 tablets every 6 hours while symptoms persist, but not more than 8 tablets in 24 hours, or as directed by a doctor
- Children under 12 years: ask a doctor
- store at room temperature
- read all product information before using
- save carton for full directions and warnings
Questions or comments: 1 877 - 466 - 0294.
Distributed by Migralex Inc. Larchmont, New York 10538 Made in Canada U.S. Patent #5538959, 5914129
TAMPER EVIDENT BOTTLE.
DO NOT USE IF INNER FOIL SEAL IMPRINTED WITH "SEALED FOR YOUR PROTECTION" IS BROKEN OR MISSING

MigralexAspirin buffered with magnesium oxide TABLET, COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!