Metronidazole
FULL PRESCRIBING INFORMATION: CONTENTS*
- Rx only
- BOXED WARNING
- METRONIDAZOLE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- METRONIDAZOLE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- GERIATRIC USE
- PEDIATRIC USE
- METRONIDAZOLE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
Rx only
BOXED WARNING
PRECAUTIONS). Unnecessary use of the drug should be avoided. Its use should be reserved for the conditions described in theINDICATIONS AND USAGEsection below.METRONIDAZOLE DESCRIPTION
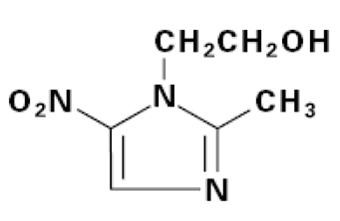
CLINICAL PHARMACOLOGY
Microbiology
Susceptibility Tests
INDICATIONS & USAGE
Symptomatic TrichomoniasisAsymptomatic Trichomoniasis
Treatment of Asymptomatic Consorts
Amebiasis
Anaerobic Bacterial Infections
METRONIDAZOLE CONTRAINDICATIONS
WARNINGS).
WARNINGS
Central and Peripheral Nervous System EffectsPRECAUTIONS
GeneralINFORMATION FOR PATIENTS
Drug Interactions).LABORATORY TESTS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS

CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
GERIATRIC USE
PEDIATRIC USE
METRONIDAZOLE ADVERSE REACTIONS
OVERDOSAGE
Treatment
DOSAGE & ADMINISTRATION
Trichomoniasis
CONTRAINDICATIONS). In pregnant patients in whom alternative treatment has been inadequate, the one-day course of therapy should not be used, as it results in higher serum levels which can reach the fetal circulation (seePRECAUTIONS, Pregnancy).
Amebiasis
Anaerobic Bacterial Infections
HOW SUPPLIED
STORAGE AND HANDLING
PROTECT FROM LIGHT
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

MetronidazoleMETRONIDAZOLE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!