METOPiRONE
Metopirone® (metyrapone USP) Capsules
FULL PRESCRIBING INFORMATION: CONTENTS*
- METOPIRONE DESCRIPTION
- METOPIRONE INDICATIONS AND USAGE
- METOPIRONE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- METOPIRONE ADVERSE REACTIONS
- OVERDOSAGE
- METOPIRONE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Metopirone ®
metyrapone USP
Capsules
Diagnostic Test of Pituitary
Adrenocorticotropic Function
Rx only
Prescribing Information
METOPIRONE DESCRIPTION
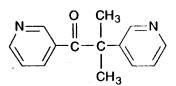
Inactive Ingredients
Pharmacodynamics
The response to Metopirone does not occur immediately. Following oral administration,
peak steroid excretion occurs during the subsequent 24-hour period.
Absorption
Metopirone is absorbed rapidly and well when administered orally as prescribed. Peak plasma concentrations are usually reached 1 hour after administration. After administration of 750 mg, mean peak plasma concentrations are 3.7 μg/mL, falling to 0.5 μg/mL 4 hours after administration. Following a single 2000-mg dose, mean peak plasma concentrations of metyrapone in plasma are 7.3 μg/mL.
Metabolism
The major biotransformation is reduction of the ketone to metyrapol, an active alcohol metabolite. Eight hours after a single oral dose, the ratio of metyrapone to metyrapol in the plasma is 1:1.5. Metyrapone and metyrapol are both conjugated with glucuronide.
Excretion
Metyrapone is rapidly eliminated from the plasma. The mean ± SD terminal elimination half‑life is 1.9 ± 0.7 hours. Metyrapol takes about twice as long as metyrapone to be eliminated from the plasma. After administration of 4.5 g metyrapone (750 mg every 4 hours), an average of 5.3% of the dose was excreted in the urine in the form of metyrapone (9.2% free and 90.8% as glucuronide) and 38.5% in the form of metyrapol (8.1% free and 91.9% as glucuronide) within 72 hours after the first dose was given.
METOPIRONE INDICATIONS AND USAGE
Metopirone is a diagnostic drug for testing hypothalamic‑pituitary ACTH function.
METOPIRONE CONTRAINDICATIONS
Metopirone is contraindicated in patients with adrenal cortical insufficiency, or hypersensitivity to Metopirone or to any of its excipients.
WARNINGS
Metopirone may induce acute adrenal insufficiency in patients with reduced adrenal
secretory capacity.
PRECAUTIONS
General
Ability of adrenals to respond to exogenous ACTH should be demonstrated before Metopirone is employed as a test. In the presence of hypo‑ or hyperthyroidism, response to the Metopirone test may be subnormal.
Since Metopirone may cause dizziness and sedation, patients should exercise caution when driving or operating machinery.
Laboratory Tests
See INTERPRETATION.
Drug Interactions
Drugs affecting pituitary or adrenocortical function, including all corticosteroid therapy, must be discontinued prior to and during testing with Metopirone.
The metabolism of Metopirone is accelerated by phenytoin; therefore, results of the test may be inaccurate in patients taking phenytoin within two weeks before. A subnormal response may occur in patients on estrogen therapy.
Metopirone inhibits the glucuronidation of acetaminophen and could possibly potentiate acetaminophen toxicity.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long‑term carcinogenicity and reproduction studies in animals have not been conducted. Metopirone was not mutagenic with or without metabolic activation in three strains of bacteria.
Pregnancy Category C
A subnormal response to Metopirone may occur in pregnant women. Animal reproduction studies have not been conducted with Metopirone. The Metopirone test was administered to 20 pregnant women in their second and third trimester of pregnancy and evidence was found that the fetal pituitary responded to the enzymatic block. It is not known if Metopirone can affect reproduction capacity. Metopirone should be given to a pregnant woman only if clearly needed.
Animal reproduction studies adequate to evaluate teratogenicity and postnatal development have not been conducted with Metopirone.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Metopirone is administered to a nursing woman.
Geriatric Use
Clinical studies of Metopirone did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
METOPIRONE ADVERSE REACTIONS
Cardiovascular System: Hypotension.
Gastrointestinal System: Nausea, vomiting, abdominal discomfort or pain.
Central Nervous System: Headache, dizziness, sedation.
Dermatologic System: Allergic rash.
Hematologic System: Rarely, decreased white blood cell count or bone marrow depression.
OVERDOSAGE
Acute Toxicity
One case has been recorded in which a 6‑year‑old girl died after two doses of Metopirone, 2 g.
Oral LD50 in animals (mg/kg): rats, 521; maximum tolerated intravenous dose in one dog, 300.
Signs and Symptoms
The clinical picture of poisoning with Metopirone is characterized by gastrointestinal symptoms and by signs of acute adrenocortical insufficiency.
Cardiovascular System: Cardiac arrhythmias, hypotension, dehydration.
Nervous System and Muscles: Anxiety, confusion, weakness, impairment of consciousness.
Gastrointestinal System: Nausea, vomiting, epigastric pain, diarrhea.
Laboratory Findings: Hyponatremia, hypochloremia, hyperkalemia.
Combined Poisoning
In patients under treatment with insulin or oral antidiabetics, the signs and symptoms of acute poisoning with Metopirone may be aggravated or modified.
Treatment
There is no specific antidote. Besides general measures to eliminate the drug and reduce its absorption, a large dose of hydrocortisone should be administered at once, together with saline and glucose infusions.
Surveillance: For a few days blood pressure and fluid and electrolyte balance should be monitored.
METOPIRONE DOSAGE AND ADMINISTRATION
Single-Dose Short Test
Interpretation
Multiple-Dose Test
Day 1: Control period - Collect 24-hour urine for measurement of 17-OHCS or 17-KGS.
Day 2: ACTH test to determine the ability of adrenals to respond - Standard ACTH test such as infusion of 50 units ACTH over 8 hours and measurement of 24-hour urinary steroids. If results indicate adequate response, the Metopirone test may proceed.
Day 3-4: Rest period.
Day 5: Administration of Metopirone: Recommended with milk or snack.
Adults: 750 mg orally, every 4 hours for 6 doses. A single dose is approximately equivalent to 15 mg/kg.
Children: 15 mg/kg orally every 4 hours for 6 doses. A minimal single dose of 250 mg is recommended.
Day 6: After administration of Metopirone - Determination of 24-hour urinary steroids for effect.
Interpretation
ACTH Test
The normal 24-hour urinary excretion of 17-OHCS ranges from 3 to 12 mg. Following continuous intravenous infusion of 50 units ACTH over a period of 8 hours, 17-OHCS excretion increases to 15 to 45 mg per 24 hours.
Metopirone
Normal response: In patients with a normally functioning pituitary, administration of Metopirone is followed by a two‑ to four‑fold increase of 17-OHCS excretion or doubling of 17-KGS excretion.
Subnormal response: Subnormal response in patients without adrenal insufficiency is indicative of some degree of impairment of pituitary function, either panhypopituitarism or partial hypopituitarism (limited pituitary reserve).
1. Panhypopituitarism is readily diagnosed by the classical clinical and chemical evidences of hypogonadism, hypothyroidism, and hypoadrenocorticism. These patients usually have subnormal basal urinary steroid levels. Depending upon the duration of the disease and degree of adrenal atrophy, they may fail to respond to exogenous ACTH in the normal manner. Administration of Metopirone is not essential in the diagnosis, but if given, it will not induce an appreciable increase in urinary steroids.
2. Partial hypopituitarism or limited pituitary reserve is the more difficult diagnosis as these patients do not present the classical signs and symptoms of hypopituitarism. Measurements of target organ functions often are normal under basal conditions. The response to exogenous ACTH is usually normal, producing the expected rise of urinary steroids (17-OHCS or 17-KGS).
The response, however, to Metopirone is subnormal; that is, no significant increase in 17‑OHCS or 17‑KGS excretion occurs.
This failure to respond to metyrapone may be interpreted as evidence of impaired pituitary‑adrenal reserve. In view of the normal response to exogenous ACTH, the failure to respond to metyrapone is inferred to be related to a defect in the CNS‑pituitary mechanisms which normally regulate ACTH secretions. Presumably the ACTH secreting mechanisms of these individuals are already working at their maximal rates to meet everyday conditions and possess limited “reserve” capacities to secrete additional ACTH either in response to stress or to decreased cortisol levels occurring as a result of metyrapone administration.
Subnormal response in patients with Cushing’s syndrome is suggestive of either autonomous adrenal tumors that suppress the ACTH-releasing capacity of the pituitary or nonendocrine ACTH-secreting tumors.
Excessive response: An excessive excretion of 17-OHCS or 17-KGS after administration of Metopirone is suggestive of Cushing’s syndrome associated with adrenal hyperplasia. These patients have an elevated excretion of urinary corticosteroids under basal conditions and will often, but not invariably, show a “supernormal” response to ACTH and also to Metopirone, excreting more than 35 mg per 24 hours of either 17-OHCS or 17-KGS.
HOW SUPPLIED
Capsules 250 mg -- soft gelatin, white to yellowish‑white, oblong, opaque, imprinted CIBA on one side and LN on the other side in brown ink.
Bottles of 18............................................................................................NDC 76336‑455‑17
Do not store above 30ºC (86ºF).
Protect from moisture and heat.
Dispense in tight container (USP).
Address medical inquiries to:
Direct Success Inc.
1710 Hwy 34
Farmingdale, NJ 07727
(855) 674-7663
(855) M-Pirone
Fax: (855) 674-6767
Manufactured by:
R.P. Scherer GmbH
Eberbach/Baden, Germany
For:
Laboratoire HRA Pharma
Paris, France
PRINCIPAL DISPLAY PANEL
Package Label - 250 mg
METOPiRONEMetyrapone CAPSULE, GELATIN COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||