Methyldopa
FULL PRESCRIBING INFORMATION: CONTENTS*
- METHYLDOPA DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- METHYLDOPA CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- METHYLDOPA ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
METHYLDOPA DESCRIPTION
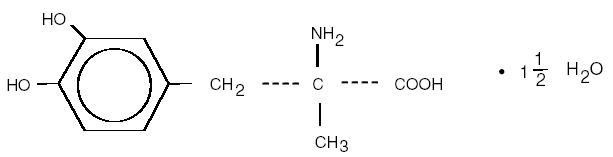
CLINICAL PHARMACOLOGY
Pharmacokinetics and Metabolism
INDICATIONS & USAGE
METHYLDOPA CONTRAINDICATIONS
WARNINGS).
WARNINGS
It is important to recognize that a positive Coombs test, hemolytic anemia, and liver disorders may occur with methyldopa therapy. The rare occurrences of hemolytic anemia or liver disorders could lead to potentially fatal complications unless properly recognized and managed. Read this section carefully to understand these reactions.PRECAUTIONS
GeneralWARNINGS).
Laboratory Tests
WARNINGS).
Drug Interactions
CONTRAINDICATIONS.
Drug/Laboratory Test Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
Teratogenic Effects
Nursing Mothers
Pediatric Use
DOSAGE AND ADMINISTRATION).
Geriatric Use
DOSAGE AND ADMINISTRATION).
METHYLDOPA ADVERSE REACTIONS
Cardiovascular:Aggravation of angina pectoris, congestive heart failure, prolonged carotid sinus hypersensitivity, orthostatic hypotension (decrease daily dosage), edema or weight gain, bradycardia.
Digestive:Pancreatitis, colitis, vomiting, diarrhea, sialadenitis, sore ortongue, nausea, constipation, distention, flatus, dryness of mouth.
Endocrine:Hyperprolactinemia.
Hematologic: Bone marrow depression, leukopenia, granulocytopenia, thrombocytopenia, hemolytic anemia; positive tests for antinuclear antibody, LE cells, and rheumatoid factor, positive Coombs test.
Hepatic:Liver disorders including hepatitis, jaundice, abnormal liver function tests (see WARNINGS).
Hypersensitivity:Myocarditis, pericarditis, vasculitis, lupus-like syndrome, drug-related fever, eosinophilia.
Nervous System/Psychiatric:Parkinsonism, Bellpalsy, decreased mental acuity, involuntary choreoathetotic movements, symptoms of cerebrovascular insufficiency, psychic disturbances including nightmares and reversible mild psychoses or depression, headache, sedation, asthenia or weakness, dizziness, light-headedness, paresthesias.
Metabolic:Rise in BUN.
Musculoskeletal:Arthralgia, with or without joint swelling; myalgia.
Respiratory:Nasal stuffiness.
Skin:Toxic epidermal necrolysis, rash.
Urogenital:Amenorrhea, breast enlargement, gynecomastia, lactation, impotence, decreased libido.
OVERDOSAGE
DOSAGE & ADMINISTRATION
AdultsPRECAUTIONS, Geriatric Use).
PEDIATRIC PATIENTS
PRECAUTIONS, Pediatric Use).
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

MethyldopaMETHYLDOPA TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!