Methocarbamol
Methocarbamol Tablets USPRx Only
FULL PRESCRIBING INFORMATION: CONTENTS*
- METHOCARBAMOL DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- METHOCARBAMOL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- METHOCARBAMOL ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
METHOCARBAMOL DESCRIPTION
11155
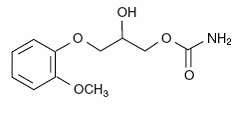
CLINICAL PHARMACOLOGY
Pharmacokinetics
Special populations
Elderly
Renally impaired
Hepatically impaired
INDICATIONS & USAGE
METHOCARBAMOL CONTRAINDICATIONS
WARNINGS
(see PRECAUTIONS, Pregnancy).
Use In Activities Requiring Mental Alertness
PRECAUTIONS
Information for Patients
Drug Interactions
See WARNINGS and PRECAUTIONS
Drug & OR Laboratory Test Interactions
Carcinogenesis & Mutagenesis & Impairment Of Fertility
Pregnancy
Teratogenic effects -Pregnancy Category C
see WARNINGS
Nursing Mothers
Pediatric Use
METHOCARBAMOL ADVERSE REACTIONS
Body as a whole
Cardiovascular system
Digestive system:
Hemic and lymphatic system:
Immune system
Nervous system:
Skin and special senses
OVERDOSAGE
Treatment
Management of overdose includes symptomatic and supportive treatment. Supportive measures include maintenance of an adequate airway, monitoring urinary output and vital signs, and administration of intravenous fluids if necessary. The usefulness of hemodialysis in managing overdose is unknown.
DOSAGE & ADMINISTRATION
HOW SUPPLIED
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense in tight container.

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
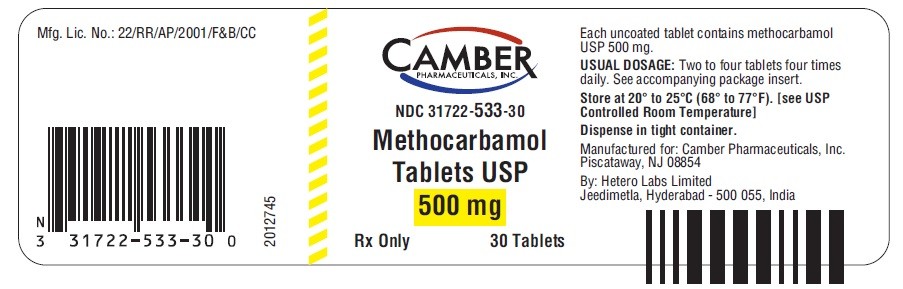

MethocarbamolMethocarbamol TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
MethocarbamolMethocarbamol TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!