Mesalamine
Prasco Laboratories
Shire US Inc.
Mesalamine Controlled-Release Capsules
FULL PRESCRIBING INFORMATION: CONTENTS*
- MESALAMINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL TRIALS
- MESALAMINE INDICATIONS AND USAGE
- MESALAMINE CONTRAINDICATIONS
- PRECAUTIONS
- Drug Interactions
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Pregnancy
- Nursing Mothers
- Pediatric Use
- MESALAMINE ADVERSE REACTIONS
- OVERDOSAGE
- MESALAMINE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
MESALAMINE DESCRIPTION
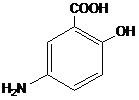
Mesalamine for oral administration is a controlled-release formulation of mesalamine, an aminosalicylate anti-inflammatory agent for gastrointestinal use. Chemically, mesalamine is 5-amino-2-hydroxybenzoic acid. It has a molecular weight of 153.14.
The structural formula is:
Each 250 mg capsule contains 250 mg of mesalamine. It also contains the following inactive ingredients: acetylated monoglyceride, castor oil, colloidal silicon dioxide, ethylcellulose, hydroxypropyl methylcellulose, starch, stearic acid, sugar, talc, and white wax. The capsule shell contains D and C Yellow #10, FD and C Blue #1, FD and C Green #3, gelatin, titanium dioxide, and other ingredients.
Each 500 mg capsule contains 500 mg of mesalamine. It also contains the following inactive ingredients: acetylated monoglyceride, castor oil, colloidal silicon dioxide, ethylcellulose, hydroxypropyl methylcellulose, starch, stearic acid, sugar, talc, and white wax. The capsule shell contains FD and C Blue #1, gelatin, titanium dioxide, and other ingredients.
CLINICAL PHARMACOLOGY
Sulfasalazine is split by bacterial action in the colon into sulfapyridine (SP) and mesalamine (5-ASA). It is thought that the mesalamine component is therapeutically active in ulcerative colitis. The usual oral dose of sulfasalazine for active ulcerative colitis in adults is 2 to 4 g per day in divided doses. Four grams of sulfasalazine provide 1.6 g of free mesalamine to the colon.
The mechanism of action of mesalamine (and sulfasalazine) is unknown, but appears to be topical rather than systemic. Mucosal production of arachidonic acid (AA) metabolites, both through the cyclooxygenase pathways, ie, prostanoids, and through the lipoxygenase pathways, ie, leukotrienes (LTs) and hydroxyeicosatetraenoic acids (HETEs), is increased in patients with chronic inflammatory bowel disease, and it is possible that mesalamine diminishes inflammation by blocking cyclooxygenase and inhibiting prostagladin (PG) production in the colon.
Human Pharmacokinetics and Metabolism
Absorption. Mesalamine Capsules are an ethylcellulose-coated, controlled-release formulation of mesalamine designed to release therapeutic quantities of mesalamine throughout the gastrointestinal tract. Based on urinary excretion data, 20% to 30% of the mesalamine is absorbed. In contrast, when mesalamine is administered orally as an unformulated 1-g aqueous suspension, mesalamine is approximately 80% absorbed.
Plasma mesalamine concentration peaked at approximately 1 mcg/mL 3 hours following a 1-g Mesalamine dose and declined in a biphasic manner. The literature describes a mean terminal half-life of 42 minutes for mesalamine following intravenous administration. Because of the continuous release and absorption of mesalamine from Mesalamine Capsules throughout the gastrointestinal tract, the true-elimination half-life cannot be determined after oral administration. N-acetylmesalamine, the major metabolite of mesalamine, peaked at approximately 3 hours at 1.8 mcg/mL, and its concentration followed a biphasic decline. Pharmacological activities of N-acetylmesalamine are unknown, and other metabolites have not been identified.
Oral mesalamine pharmacokinetics were nonlinear when Mesalamine capsules were dosed from 250 mg to 1 g four times daily, with steady-state mesalamine plasma concentrations increasing about nine times, from 0.14 mcg/mL to 1.21 mcg/mL, suggesting saturable first-pass metabolism. N-acetylmesalamine pharmacokinetics were linear.
Elimination. About 130 mg free mesalamine was recovered in the feces following a single 1-g Mesalamine dose, which was comparable to the 140 mg of mesalamine recovered from the molar equivalent sulfasalazine tablet dose of 2.5 g. Elimination of free mesalamine and salicylates in feces increased proportionately with Mesalamine dose. N-acetylmesalamine was the primary compound excreted in the urine (19% to 30%) following Mesalamine dosing.
CLINICAL TRIALS
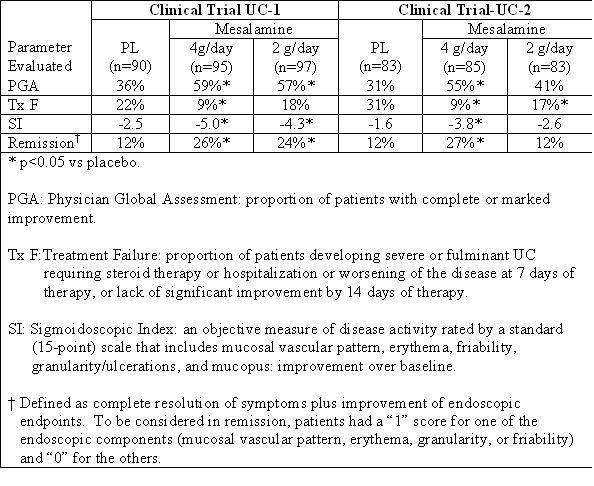
In two randomized, double-blind, placebo-controlled, dose-response trials (UC-1 and UC-2) of 625 patients with active mild to moderate ulcerative colitis, Mesalamine, at an oral dose of 4 g/day given 1 g four times daily, produced consistent improvement in prospectively identified primary efficacy parameters, PGA, Tx F, and SI as shown in the table below.
The 4-g dose of Mesalamine also gave consistent improvement in secondary efficacy parameters, namely the frequency of trips to the toilet, stool consistency, rectal bleeding, abdominal/rectal pain, and urgency. The 4-g dose of Mesalamine induced remission as assessed by endoscopic and symptomatic endpoints. In some patients, the 2-g dose of Mesalamine was observed to improve efficacy parameters measured. However, the 2-g dose gave inconsistent results in primary efficacy parameters across the two adequate and well-controlled trials.
MESALAMINE INDICATIONS AND USAGE
Mesalamine is indicated for the induction of remission and for the treatment of patients with mildly to moderately active ulcerative colitis.
MESALAMINE CONTRAINDICATIONS
Mesalamine is contraindicated in patients who have demonstrated hypersensitivity to mesalamine, any other components of this medication, or salicylates.
PRECAUTIONS
General
Caution should be exercised if Mesalamine is administered to patients with impaired hepatic function.
Mesalamine has been associated with an acute intolerance syndrome that may be difficult to distinguish from a flare of inflammatory bowel disease. Although the exact frequency of occurrence cannot be ascertained, it has occurred in 3% of patients in controlled clinical trials of mesalamine or sulfasalazine. Symptoms include cramping, acute abdominal pain and bloody diarrhea, sometimes fever, headache, and rash. If acute intolerance syndrome is suspected, prompt withdrawal is required. If a rechallenge is performed later in order to validate the hypersensitivity, it should be carried out under close medical supervision at reduced dose and only if clearly needed.
Renal
Caution should be exercised if Mesalamine is administered to patients with impaired renal function. Single reports of nephrotic syndrome and interstitial nephritis associated with mesalamine therapy have been described in the foreign literature. There have been rare reports of interstitial nephritis in patients receiving Mesalamine. In animal studies, a 13-week oral toxicity study in mice and 13-week and 52-week oral toxicity studies in rats and cynomolgus monkeys have shown the kidney to be the major target organ of mesalamine toxicity. Oral daily doses of 2400 mg/kg in mice and 1150 mg/kg in rats produced renal lesions including granular and hyaline casts, tubular degeneration, tubular dilation, renal infarct, papillary necrosis, tubular necrosis, and interstitial nephritis. In cynomolgus monkeys, oral daily doses of 250 mg/kg or higher produced nephrosis, papillary edema, and interstitial fibrosis. Patients with preexisting renal disease, increased BUN or serum creatinine, or proteinuria should be carefully monitored, especially during the initial phase of treatment. Mesalamine-induced nephrotoxicity should be suspected in patients developing renal dysfunction during treatment.
Drug Interactions
There are no data on interactions between Mesalamine and other drugs.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 104-week dietary carcinogenicity study of mesalamine, CD-1 mice were treated with doses up to 2500 mg/kg/day and it was not tumorigenic. For a 50 kg person of average height (1.46 m2 body surface area), this represents 2.5 times the recommended human dose on a body surface area basis (2960 mg/m2/day). In a 104-week dietary carcinogenicity study in Wistar rats, mesalamine up to a dose of 800 mg/kg/day was not tumorigenic. This dose represents 1.5 times the recommended human dose on a body surface area basis.
No evidence of mutagenicity was observed in an in vitro Ames test and in an in vivo mouse micronucleus test.
No effects on fertility or reproductive performance were observed in male or female rats at oral doses of mesalamine up to 400 mg/kg/day (0.8 times the recommended human dose based on body surface area).
Semen abnormalities and infertility in men, which have been reported in association with sulfasalazine, have not been seen with Mesalamine capsules during controlled clinical trials.
Pregnancy
Category B. Reproduction studies have been performed in rats at doses up to 1000 mg/kg/day (5900 mg/M2) and rabbits at doses of 800 mg/kg/day (6856 mg/M2) and have revealed no evidence of teratogenic effects or harm to the fetus due to mesalamine. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, Mesalamine should be used during pregnancy only if clearly needed.
Mesalamine is known to cross the placental barrier.
Nursing Mothers
Minute quantities of mesalamine were distributed to breast milk and amniotic fluid of pregnant women following sulfasalazine therapy. When treated with sulfasalazine at a dose equivalent to 1.25 g/day of mesalamine, 0.02 mcg/mL to 0.08 mcg/mL and trace amounts of mesalamine were measured in amniotic fluid and breast milk, respectively. N-acetylmesalamine, in quantities of 0.07 mcg/mL to 0.77 mcg/mL and 1.13 mcg/mL to 3.44 mcg/mL, was identified in the same fluids, respectively.
Caution should be exercised when Mesalamine is administered to a nursing woman.
No controlled studies with Mesalamine during breast-feeding have been carried out. Hypersensitivity reactions like diarrhea in the infant cannot be excluded.
Pediatric Use
Safety and efficacy of Mesalamine in pediatric patients have not been established.
MESALAMINE ADVERSE REACTIONS
In combined domestic and foreign clinical trials, more than 2100 patients with ulcerative colitis or Crohn's disease received Mesalamine therapy. Generally, Mesalamine therapy was well tolerated. The most common events (ie, greater than or equal to 1%) were diarrhea (3.4%), headache (2.0%), nausea (1.8%), abdominal pain (1.7%), dyspepsia (1.6%), vomiting (1.5%), and rash (1.0%).
In two domestic placebo-controlled trials involving over 600 ulcerative colitis patients, adverse events were fewer in Mesalamine-treated patients than in the placebo group (Mesalamine 14% vs placebo 18%) and were not dose-related. Events occurring at 1% or more are shown in the table below. Of these, only nausea and vomiting were more frequent in the Mesalamine group. Withdrawal from therapy due to adverse events was more common on placebo than Mesalamine (7% vs 4%).
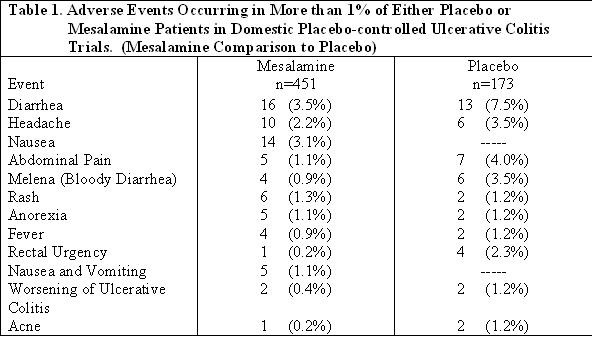
Clinical laboratory measurements showed no significant abnormal trends for any test, including measurement of hematological, liver, and kidney function.
The following adverse events, presented by body system, were reported infrequently (ie, less than 1%) during domestic ulcerative colitis and Crohn's disease trials. In many cases, the relationship to Mesalamine has not been established.
Gastrointestinal: abdominal distention, anorexia, constipation, duodenal ulcer, dysphagia, eructation, esophageal ulcer, fecal incontinence, GGTP increase, GI bleeding, increased alkaline phosphatase, LDH increase, mouth ulcer, oral moniliasis, pancreatitis, rectal bleeding, SGOT increase, SGPT increase, stool abnormalities (color or texture change), thirst
Dermatological: acne, alopecia, dry skin, eczema, erythema nodosum, nail disorder, photosensitivity, pruritus, sweating, urticaria
Nervous System: depression, dizziness, insomnia, somnolence, paresthesia
Cardiovascular: palpitations, pericarditis, vasodilation
Other: albuminuria, amenorrhea, amylase increase, arthralgia, asthenia, breast pain, conjunctivitis, ecchymosis, edema, fever, hematuria, hypomenorrhea, Kawasaki-like syndrome, leg cramps, lichen planus, lipase increase, malaise, menorrhagia, metrorrhagia, myalgia, pulmonary infiltrates, thrombocythemia, thrombocytopenia, urinary frequency
One week after completion of an 8-week ulcerative colitis study, a 72-year-old male, with no previous history of pulmonary problems, developed dyspnea. The patient was subsequently diagnosed with interstitial pulmonary fibrosis without eosinophilia by one physician and bronchiolitis obliterans with organizing pneumonitis by a second physician. A causal relationship between this event and mesalamine therapy has not been established.
Published case reports and/or spontaneous postmarketing surveillance have described infrequent instances of pericarditis, fatal myocarditis, chest pain and T-wave abnormalities, hypersensitivity pneumonitis, pancreatitis, nephrotic syndrome, interstitial nephritis, hepatitis, aplastic anemia, pancytopenia, leukopenia, agranulocytosis, or anemia while receiving mesalamine therapy. Anemia can be a part of the clinical presentation of inflammatory bowel disease. Allergic reactions, which could involve eosinophilia, can be seen in connection with Mesalamine therapy.
Postmarketing Reports
The following events have been identified during post-approval use of mesalamine in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of seriousness, frequency of reporting, or potential causal connection to mesalamine:
Gastrointestinal: Reports of hepatoxicity, including elevated liver enzymes (SGOT/AST, SGPT/ALT, GGT, LDH, alkaline phosphatase, bilirubin), hepatitis, jaundice, cholestatic jaundice, cirrhosis, and possible hepatocellular damage including liver necrosis and liver failure. Some of these cases were fatal. One case of Kawasaki-like syndrome which included hepatic function changes was also reported.
Other: Postmarketing reports of pneumonitis, granulocytopenia, systemic lupus erythematosis, acute renal failure, and chronic renal failure have been received in patients taking Mesalamine.
OVERDOSAGE
Single oral doses of mesalamine up to 5 g/kg in pigs or a single intravenous dose of mesalamine at 920 mg/kg in rats were not lethal.
There is no clinical experience with Mesalamine overdosage. Mesalamine is an aminosalicylate, and symptoms of salicylate toxicity may be possible, such as: tinnitus, vertigo, headache, confusion, drowsiness, sweating, hyperventilation, vomiting, and diarrhea. Severe intoxication with salicylates can lead to disruption of electrolyte balance and blood-pH, hyperthermia, and dehydration.
Treatment of Overdosage. Since Mesalamine is an aminosalicylate, conventional therapy for salicylate toxicity may be beneficial in the event of acute overdosage. This includes prevention of further gastrointestinal tract absorption by emesis and, if necessary, by gastric lavage. Fluid and electrolyte imbalance should be corrected by the administration of appropriate intravenous therapy. Adequate renal function should be maintained.
MESALAMINE DOSAGE AND ADMINISTRATION
The recommended dosage for the induction of remission and the symptomatic treatment of mildly to moderately active ulcerative colitis is 1g (4 Mesalamine 250 mg capsules or 2 Mesalamine 500 mg capsules) 4 times a day for a total daily dosage of 4g. Treatment duration in controlled trials was up to 8 weeks.
HOW SUPPLIED
Mesalamine controlled-release 250 mg capsules are supplied in bottles of 240 capsules (NDC 66993-410-24). Each green and blue capsule contains 250 mg of mesalamine in controlled-release beads. Mesalamine controlled-release capsules are identified with a pentagonal starburst logo and the number 2010 on the green portion and S429 250 mg on the blue portion of the capsules.
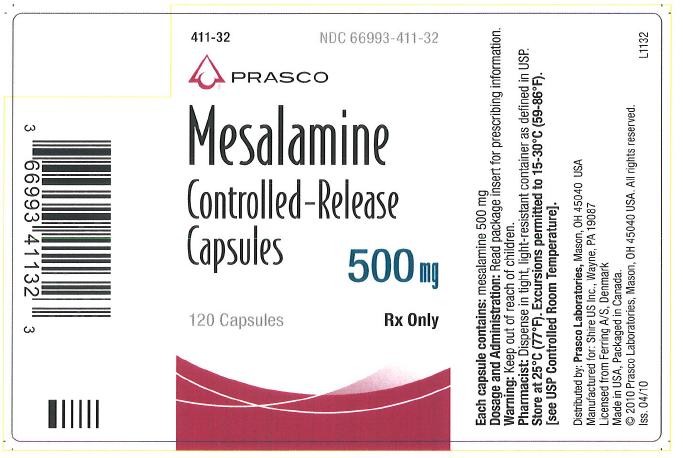
Mesalamine controlled-release 500 mg capsules are supplied in bottles of 120 capsules (NDC 66993-411-32). Each blue capsule contains 500 mg of mesalamine in controlled-release beads. Mesalamine controlled-release capsules are identified with a pentagonal starburst logo and S429 500 mg on the capsules.
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature].

Distributed by:
Prasco Laboratories
Mason, OH 45040 USA
Manufactured for:
Shire US Inc.
Wayne, PA 19087
Licensed from Ferring A/S, Denmark
© 2010 Prasco Laboratories
N4100
Iss. 06/2008
PRINCIPAL DISPLAY PANEL
NDC 66993-410-24 - 250 mg bottle of 240 capsules
NDC 66993-411-32 - 500 mg bottle of 120 capsules
Mesalaminemesalamine CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Mesalaminemesalamine CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||