Mentholatum Pain Relief Deep Heating
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients
- Purpose
- Mentholatum Pain Relief Deep Heating Uses
- Warnings
- If pregnant or breast-feeding,
- Keep Out of Reach of Children.
- Directions
- Inactive ingredients
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredients
Menthol 6%
Methyl salicylate 20%
Purpose
Menthol - Topical analgesic
Methyl salicylate - Topical analgesic
Mentholatum Pain Relief Deep Heating Uses
temporarily relieves minor aches and pains of muscles and joints due to
- arthritis
- simple backache
- strains
- sprains
Warnings
For external use only
When using this product
- use only as directed
- do not get into eyes or on mucous membranes
- do not apply to wounds or to damaged skin
- do not bandage tightly
- do not use with a heating pad or apply external heat
- do not use in combination with other external analgesic products
Stop use and ask a doctor if
- condition worsens
- excessive irritation, burning or discomfort of the skin develops
- symptoms persist for more than 7 days or clear up and occur again within a few days
If pregnant or breast-feeding,
ask a healthcare professional before use.
Keep Out of Reach of Children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years and over: apply to affected area not more than 3 to 4 times daily
- children under 12 years: ask a doctor
Inactive ingredients
carbomer, lanolin oil, polysorbate 60, purified water, sorbitan monosterate, trolamine
Package/Label Principal Display Panel
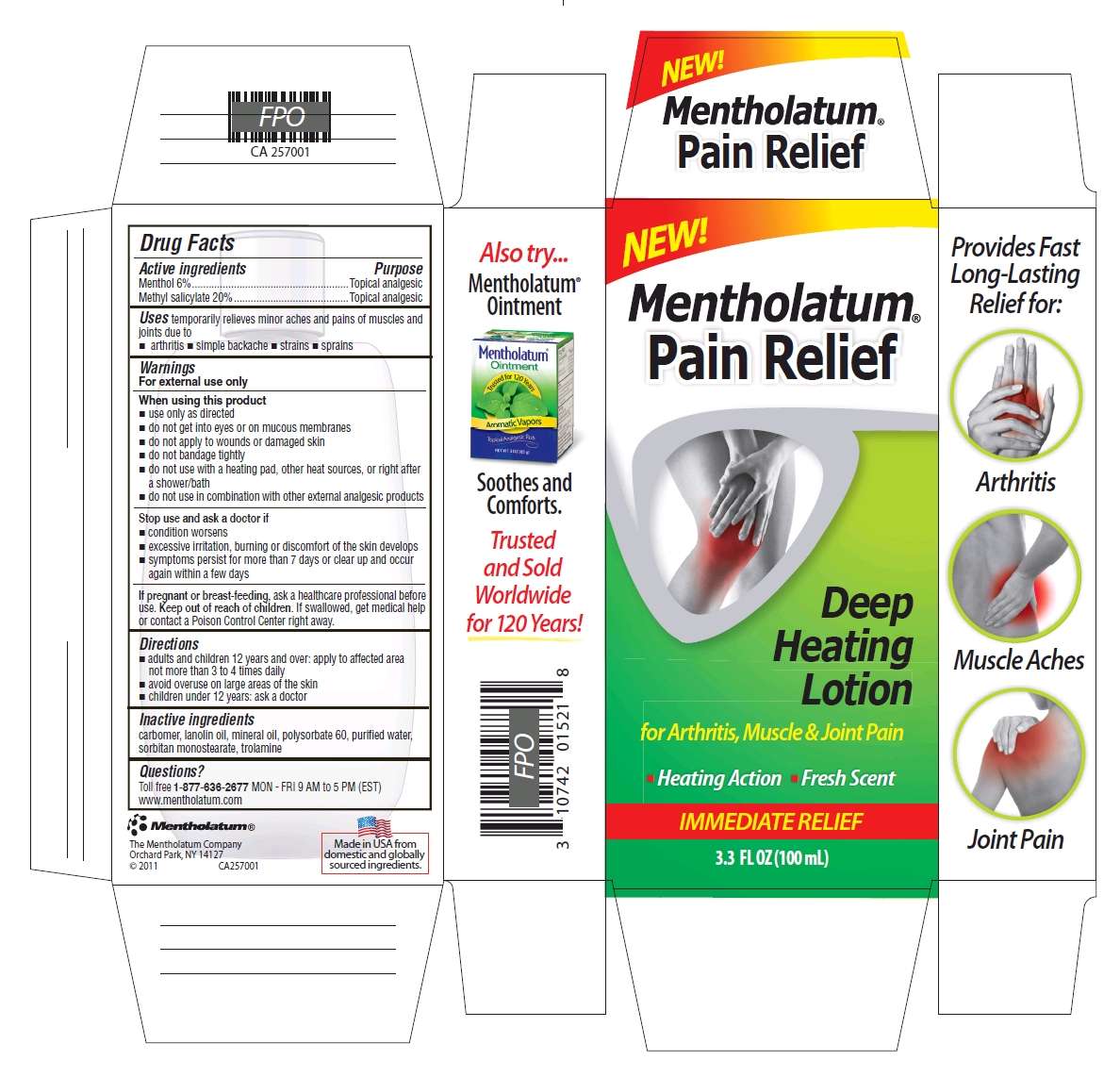
The Mentholatum Company
Orchard Park, NY 14127
Mentholatum Pain Relief Deep Heatingmenthol, methyl salicylate LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!