Menthol and Zinc Oxide
Dynarex Corporation
Dynarex Corporation
Calasoothe Ointment
FULL PRESCRIBING INFORMATION: CONTENTS*
- Purpose:
- Warnings:
- Indications & Usage
- Dosage & Administration:
- Keep Out Of Reach Of Children
- Inactive Ingredients
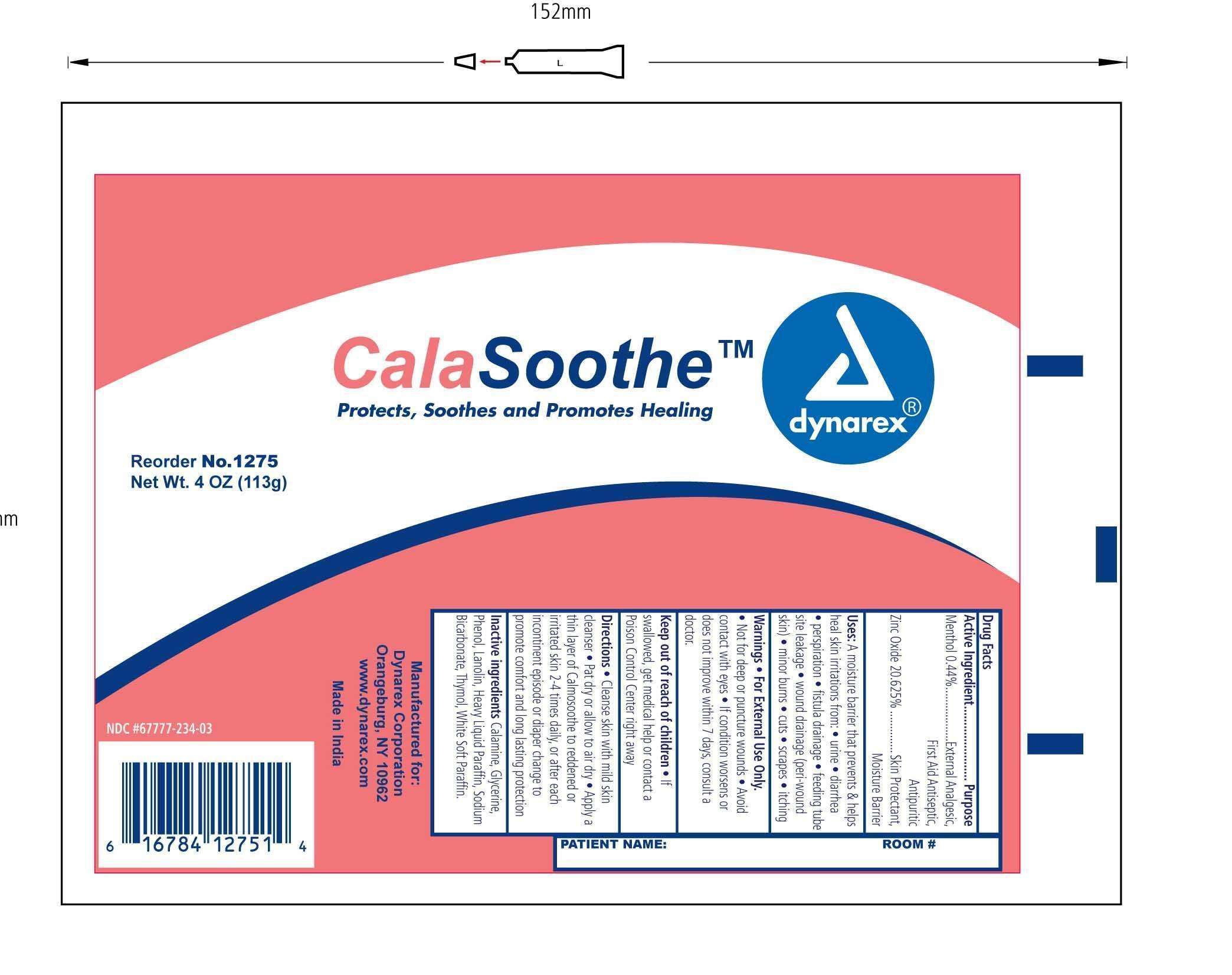
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient Purpose
Menthol 0.44% v/v External Analgesic
First Aid Antiseptic
Antipuritic
Zinc Oxide 20.625% v/v Skin Protectant
Moisture Barrier
Purpose:
A moisture barrier that prevents and helps heal skin irritations from;
- urine
- diarrhea
- perspiration
- fistula drainage
- feeding tube site leakage
- wound drainage (peri-wound skin)
- minor burns
- cuts
- scrapes
- itching
Warnings:
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
Indications & Usage
- Not for deep or puncture wounds.
- Avoid contact with eyes.
- If condition worsens or does not improve within 7 days, consult a doctor
Dosage & Administration:
- Clean skin with mild skin cleanser
- Pat dry or allow to air dry
- Apply a thin layer of Calasoothe to reddened or irritated skin 2-4 times daily, or after each incontinent episode or diaper change to promote comfort and long lasting protection
Keep Out Of Reach Of Children
Keep Out Of Reach Of Children
If swallowed , get medical help or contact a Poison Control Center right away.
Inactive Ingredients
Inactive Ingredients: Calamine, Glycerine, Phenol, Lanolin, Heavy Liquid Paraffin, Sodium Bicarbonate, Thymol, White Soft Paraffin
Principal Display Panel
Principal Display Panel - Calasoothe_Ointment
Calasoothe_Ointment.jpg
Menthol and Zinc OxideMenthol and Zinc Oxide OINTMENT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||