MembraneBlue
Dutch Ophthalmic Research Center International B.V.
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MembraneBlue™ 0.15% safely and effectively. See full prescribing information for MembraneBlue™ 0.15%. MembraneBlue™ 0.15% (trypan blue ophthalmic solution) Initial U.S. Approval: 2004 INDICATIONS AND USAGE For use as an aid in ophthalmic posterior surgery; Facilitating removal of epiretinal tissue. (1) DOSAGE AND ADMINISTRATION Prior to injection of MembraneBlue™ 0.15% perform a ‘fluid-air exchange’; Carefully apply MembraneBlue™ 0.15% to epiretinal membranes using a blunt cannula; Remove all excess dye; Or Inject MembraneBlue™ 0.15% directly in a BSS filled - vitreous cavity; Wait 30 seconds; Remove all excess dye (2) DOSAGE FORMS AND STRENGTHS MembraneBlue™ 0.15% (trypan blue ophthalmic solution) in a volume of 0.5 mL. (3) CONTRAINDICATIONS Insertion of a non-hydrated (dry state), hydrophilic acrylic intraocular lens (IOL). (4) WARNINGS AND PRECAUTIONS Excessive staining: Excess MembraneBlue™ 0.15% should be removed from the eye immediately after staining. Priming of the syringe: Make sure the plunger moves smoothly before use: first retract the plunger or twist the plunger in a clockwise motion before injecting the fluid. (5) Side Effects Discoloration of high water content hydrogen intraocular lenses; Inadvertent staining of the posterior lens capsule and vitreous face; (6) to report suspected adverse reactions contact Dutch Ophthalmic, USA at 1-800-75-DUTCH or FDA at 1-800-FDA-1088 or www.fda.gov/medwatchUSE IN SPECIFIC POPULATIONSTrypan blue should not be given to pregnant women; (8)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1. MembraneBlue Indications and Usage
- 2. Dosage and Administration
- 3. Dosage Forms and Strength
- 4. Contraindications
- 5. Warnings and Precautions
- 6. Side Effects
- 8. Use in Specific Populations
- 11. Description
- 12. Clinical Pharmacology
- 13. Nonclinical Toxicology
- 16. How Supplied/Storage and Handling
FULL PRESCRIBING INFORMATION
1. Indications and Usage
MembraneBlue™ 0.15% is indicated for use as an aid in ophthalmic surgery by staining the epiretinal membranes during ophthalmic surgical vitrectomy procedures, facilitating removal of the tissue.
2. Dosage and Administration
Make sure the plunger moves smoothly before use. Prime the syringe prior to use by retracting the plunger before injecting the fluid. Alternatively, twist the plunger into the stopper in a clockwise motion until tight. Once tight, continue turning the plunger in a clockwise motion until the stopper rotates freely within the syringe, two or three rotations. The syringe is now primed and suitable for injection.
Before injection of MembraneBlue™ 0.15% perform a ‘fluid-air exchange’, i.e. filling the entire vitreous cavity with air, to prevent aqueous dilution of MembraneBlue™ 0.15%. MembraneBlue™ 0.15% is carefully applied to the retinal membrane using a blunt cannula attached to the MembraneBlue™ 0.15% syringe, without allowing the cannula to contact or damage the retina. Sufficient staining is expected on contact with the membrane. All excess dye should be removed from the vitreous cavity before performing an air-fluid exchange, to prevent unnecessary spreading of the dye.
MembraneBlue™ 0.15% can also be injected directly in a BSS filled vitreous cavity (instead of injecting under air). Clinical use demonstrated that, after complete vitreous and posterior hyaloid removal, sufficient staining is achieved after 30 seconds of application under BSS.
MembraneBlue™ 0.15% is intended to be applied directly on the areas where membranes could be present, staining any portion of the membrane which comes in contact with the dye. The dye does not penetrate the membrane.
3. Dosage Forms and Strength
MembraneBlue™ 0.15% (trypan blue ophthalmic solution) is supplied in 2.25 mL syringes filled to a volume of 0.5 mL.
4. Contraindications
MembraneBlue™ 0.15% is contraindicated when a non-hydrated (dry state), hydrophilic acrylic intraocular lens (IOL) is planned to be inserted into the eye. The dye may be absorbed by the IOL and stain it.
5. Warnings and Precautions
Excessive staining:
It is recommended that after injection all excess MembraneBlue™ 0.15% be immediately removed from the eye.
Priming of the syringe:
Make sure the plunger moves smoothly before use: first retract the plunger or twist the plunger in a clockwise motion before injecting the fluid.
6. Side Effects
Adverse reactions reported following use of MembraneBlue™ 0.15% include discoloration of high water content hydrogen intraocular lenses (see Contraindications) and inadvertent staining of the posterior lens capsule and vitreous face. Staining of the posterior lens capsule or staining of the vitreous face is generally self limited, lasting up to one week.
8. Use in Specific Populations
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category C. Trypan blue is teratogenic in rats, mice, rabbits, hamsters, dogs, guinea pigs, pigs, and chickens. The majority of teratogenicity studies performed involve intravenous, intraperitoneal, or subcutaneous administration in the rat. The teratogenic dose is 50 mg/kg as a single dose or 25 mg/kg/day during embryogenesis in the rat. These doses are approximately 4,000- and 2,000-fold the maximum recommended human dose of 0.75 mg per injection based in a 60 kg person, assuming that the whole dose is completely absorbed. Characteristic anomalies included neural tube, cardiovascular, vertebral, tail, and eye defects. Trypan blue also caused an increase in post-implantation mortality, and decreased fetal weight. In the monkey, trypan blue caused abortions with single or two daily doses of 50 mg/kg between 20th to 25th days of pregnancy, but no apparent increase in birth defects (approximately 4,000-fold maximum recommended human dose of 0.75 mg per injection, assuming total absorption). There are no adequate and well-controlled studies in pregnant women. Trypan blue should be given to a pregnant woman only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when trypan blue is administered to a nursing woman.
8.4 Pediatric use
The safety and effectiveness of trypan blue have been established in pediatric patients. Use of trypan blue is supported by evidence from an adequate and well-controlled study in pediatric patients.
8.5 Geriatric use
No overall differences in safety and effectiveness have been observed between elderly and younger patients.
11. Description
MembraneBlue™ 0.15% (trypan blue ophthalmic solution) is a sterile solution of trypan blue (an acid di-azo group dye). MembraneBlue™ 0.15% selectively stains epiretinal membranes during ophthalmic surgical vitrectomy procedures.
Each mL of MembraneBlue™ 0.15% contains: 1.5 mg trypan blue; 1.9 mg sodium mono-hydrogen orthophosphate (Na2HPO4.2H2O); 0.3 mg sodium di-hydrogen orthophosphate (NaH2PO4.2H2O); 8.2 mg sodium chloride (NaCl); and water for injection. The pH is 7.3 - 7.6. The osmolality is 257-314 mOsm/kg.
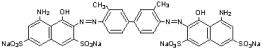
The drug substance trypan blue has the chemical name 3,3’-[(3,3’-dimethyl-4,4’-biphenylylene) bis (azo)] bis(5-amino-4-hydroxy-2,7-naphthalenedisulfonic acid) tetra sodium salt, a molecular weight of 960.8, a molecular formula of C34H24N6Na4O14S4, and has the following chemical structure:
12. Clinical Pharmacology
12.1 Mechanism of Action
MembraneBlue™ 0.15% selectively stains membranes in the human eye during posterior surgery, such as epiretinal membranes (ERM) and Internal Limiting Membranes (ILM).
13. Nonclinical Toxicology
Trypan blue is carcinogenic in rats. Wister/Lewis rats developed lymphomas after receiving subcutaneous injections of 1% trypan blue dosed at 50 mg/kg every other week for 52 weeks (total dose approximately 100,000-fold the maximum recommended human dose of 0.75 mg per injection in a 60 kg person, assuming total absorption).
Trypan blue was mutagenic in the Ames test and caused DNA strand breaks in vitro.
16. How Supplied/Storage and Handling
MembraneBlue™ 0.15% is supplied as follows:
0.5 mL of MembraneBlue™ 0.15% in a sterile single-use Luer Lok, 2.25 mL glass syringe, grey rubber plunger stopper and tip cap with polypropylene plunger rod in a peel pouch. Five pouched products are packed in one distribution box.
MembraneBlue™ 0.15% is stored at 15-25ºC (59-77ºF). Protect from direct sunlight.
Rx ONLY
Manufactured by
D.O.R.C. International b.v.
Scheijdelveweg 2
3214 VN Zuidland
The Netherlands
Distributed in the United States by
Dutch Ophthalmic, USA
10 Continental Drive, Bldg 1
Exeter, NH 03833, USA
Phone: 800-75-DUTCH or 603-778-6929
US Patents 6,696,430 and 6,372,449 Copyright ©, 2009 Dutch Ophthalmic Research Center
MembraneBlueTrypan Blue INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||