Medicaine Sting and Bite
James Alexander Corporation
James Alexander Corporation
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Purpose
analgesic
for external use only
do not use in the eyes
not for prolonged use
stop use and ask a doctor if condition for this preparation is used persists, or if a rash or irritation develops.
Uses
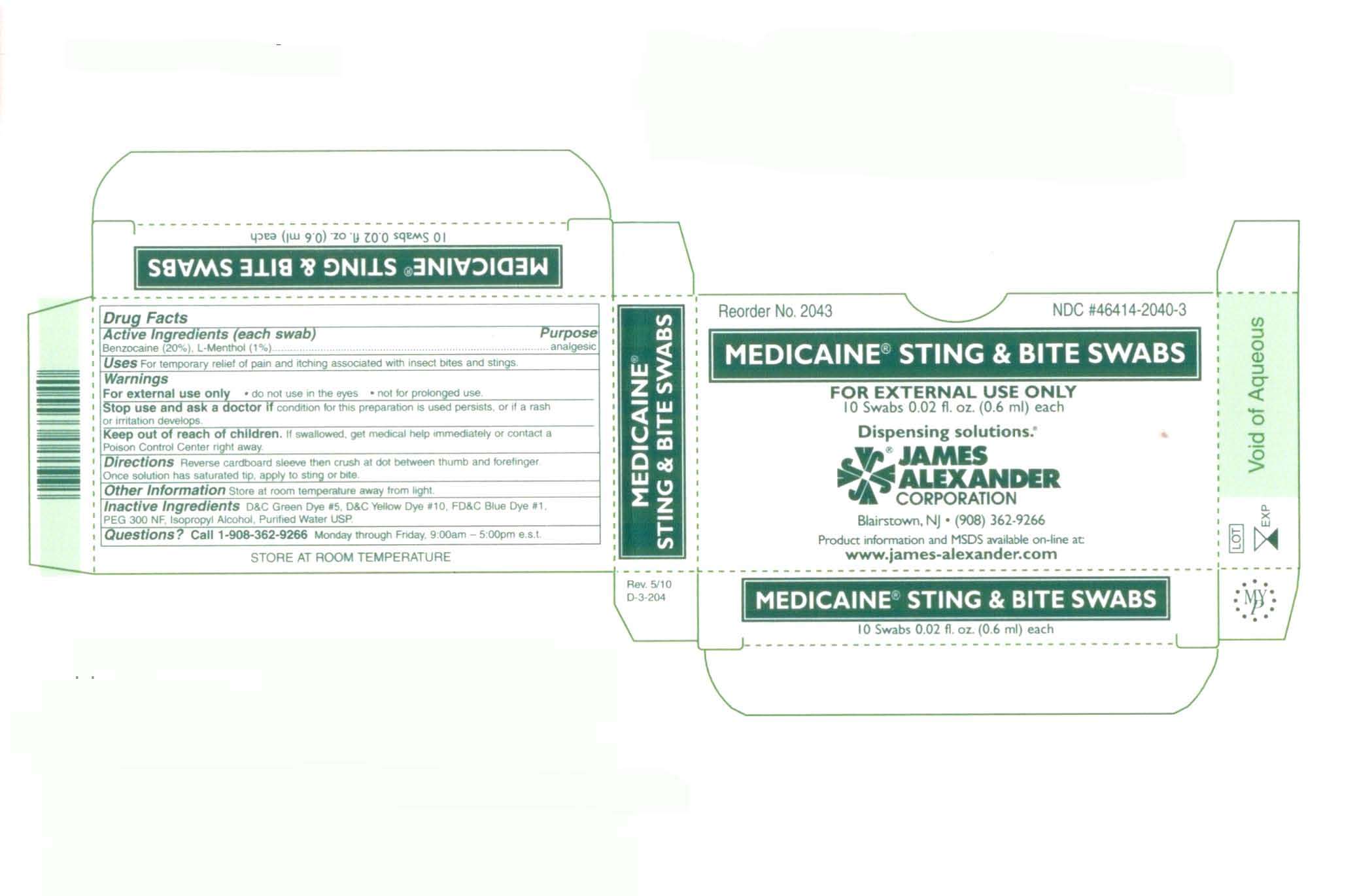
Medicaine Sting and BiteBenzocaine SWAB
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!