Mary Kay Tinted Moisturizer Sunscreen SPF 20 Beige 1
Mary Kay Tinted Moisturizer SPF 20 Beige 1
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Warnings
- Indications:
- Directions:
- OTHER INGREDIENTS/AUTRES INGREDIENTS:
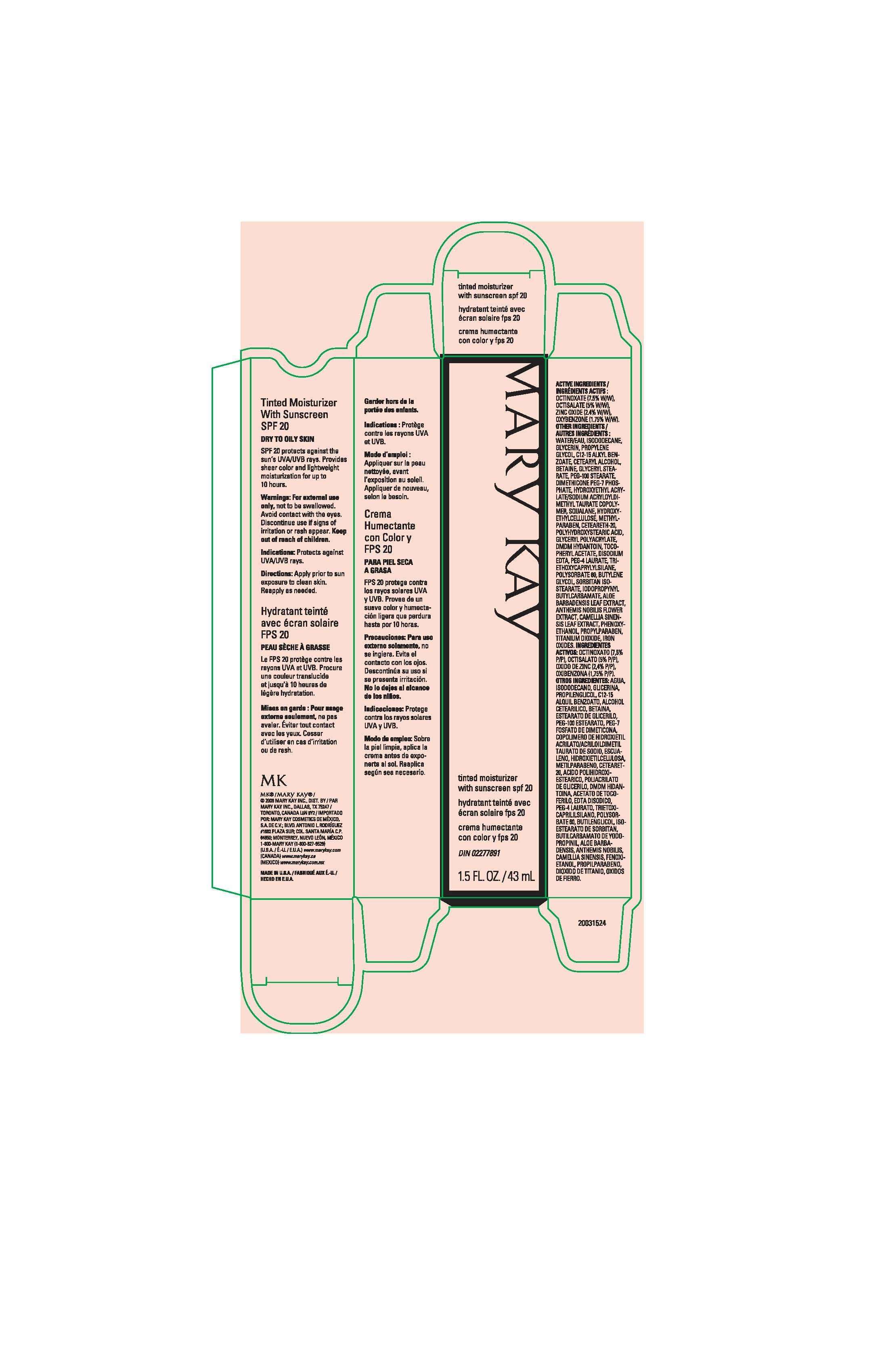
- Principal Display Panel - 43 ml carton
FULL PRESCRIBING INFORMATION
Active Ingredients
Octinoxate (7.5% W/W)
Octisalate (5% W/W)
Zinc Oxide (2.4% W/W)
Oxybenzone (1.75% W/W)
Warnings
For external use only, not to be swallowed. Avoid contact with the eyes.
Discontinue use
if signs of irritation or rash appear.
Keep out of reach of children.
Indications:
Protects against UVA/UVB rays.
Directions:
Apply prior to sun exposure to clean skin. Reapply as needed.
OTHER INGREDIENTS/AUTRES INGREDIENTS:
WATER/EAU, ISODODECANE, GLYCERIN, PROPYLENE GLYCOL, C12-15 ALKYL BENZOATE, CETEARYL ALCOHOL, BETAINE, GLYCERYL STEARATE, PEG-100 STEARATE, DIMETHICONE PEG-7 PHOSPHATE, HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER, SQUALANE, HYDROXYETHYLCELLULOSE, METHYLPARABEN, CETEARETH-20, POLYHYDROXYSTEARIC ACID, GLYCERYL POLYACRYLATE, DMDM HYDANTOIN, TOCOPHERYL ACETATE, DISODIUM EDTA, PEG-4 LAURATE, TRIETHOXYCAPRYLYLSILANE, POLYSORBATE-60, BUTYLENE GLYCOL, SORBITAN ISOSTEARATE, IODOPROPYNYL BUTYLCARBAMATE, ALOE BARBADENSIS LEAF EXTRACT, ANTHEMIS NOBILIS FLOWER EXTRACT, CAMELLIA SINENSIS LEAF EXTRACT, PHENOXYETHANOL, PROPYLPARABEN, TITANIUM DIOXIDE, IRON OXIDES.
Principal Display Panel - 43 ml carton
Mary Kay
tinted moisturizer with sunscreen spf 20
hydratant teinte avec ecran solaire fps 20
crema humectante con color y fps 20
1.5 FL. OZ. / 43 mL
Mary Kay Tinted Moisturizer Sunscreen SPF 20 Beige 1octinoxate, octisalate, zinc oxide, oxybenzone, CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||