Mamonde Total
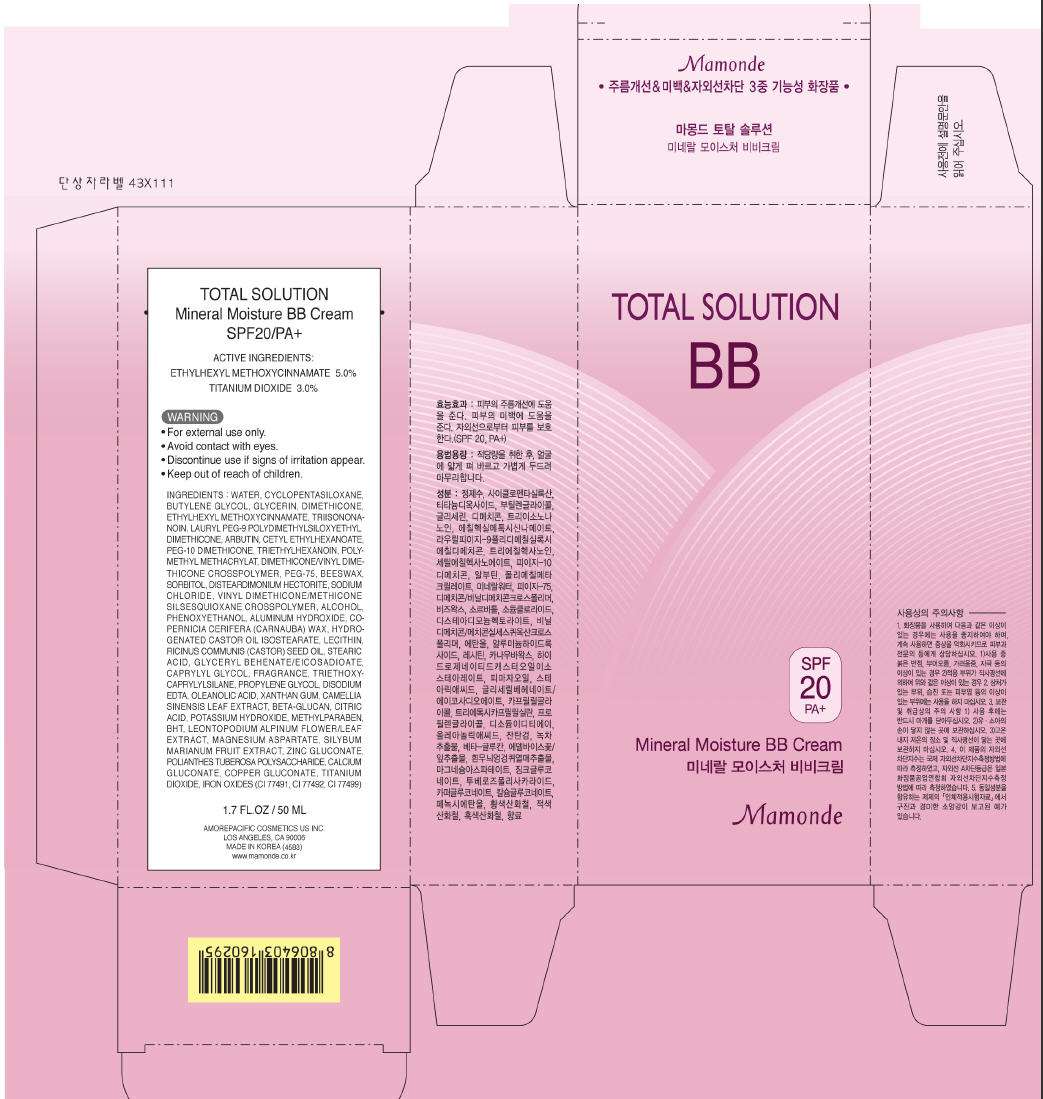
Mamonde TOTAL SOLUTION Mineral Moisture BB Cream SPF20/PA+
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENTS
ETHYLHEXYL METHOXYCINNAMATE 5.0%
TITANIUM DIOXIDE 3.0%
WARNING
- For external use only.
- Avoid contact with eyes.
- Discontinue use if signs of irritation appear.
- Keep out of reach of children.
INGREDIENTS
WATER, CYCLOPENTASILOXANE, BUTYLENE GLYCOL, GLYCERIN, DIMETHICONE, ETHYLHEXYL METHOXYCINNAMATE, TRIISONONANOIN, LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, ARBUTIN, CETYL ETHYLHEXANOATE, PEG-10 DIMETHICONE, TRIETHYLHEXANOIN, POLYMETHYL METHACRYLAT, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, PEG-75, BEESWAX, SORBITOL, DISTEARDIMONIUM HECTORITE, SODIUM CHLORIDE, VINYL DIMETHICONE/METHICONE SILSEQUIOXANE CROSSPOLYMER, ALCOHOL, PHENOXYETHANOL, ALUMINUM HYDROXIDE, COPERNICIA CERIFERA (CARNUBA) WAX, HYDROGENATED CASTOR OIL ISOSTEARATE, LECITHIN, RICINUS COMMUNIS (CASTOR) SEED OIL, STEARIC ACID, GLYCERYL BEHENATE/EICOSADIOATE, CAPRYLYL GLYCOL, FRAGRANCE, TRIETHOXYCAPRYLYLSILANE, PROPYLENE GLYCOL, DISODIUM EDTA, OLEANOLIC ACID, XANTHAN GUM, CAMELLIA SINENSIS LEAF EXTRACT, BETA-GLUCAN, CITRIC ACID, POTASSIUM HYDROXIDE, METHYLPARABEN, BHT, LEONTOPODIUM ALPINUM FLOWER/LEAF EXTRACT, MAGNESIUM ASPARTATE, SILYBUM MARIANUM FRUIT EXTRACT, ZINC GLUCONATE, POLIANTHES TUBEROSA POLYSACCHARIDE, CALCIUM GLUCONATE, COPPER GLUCONATE, TITANIUM DIOXIDE, IRON OXIDES (CI 77491, CI77492, CI77499)
1.7 FL.OZ / 50 ML
AMOREPACIFIC COSMETICS US INC.
LOS ANGELES, CA 90006
MADE IN KOREA (4583)
www.mamonde.co.kr
PRINCIPAL DISPLAY PANEL - 50 ML Carton
TOTAL SOLUTION
BB
SPF
20
PA+
Mineral Moisture BB Cream
Mamonde
Mamonde TotalOCTINOXATE and TITANIUM DIOXIDE CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||