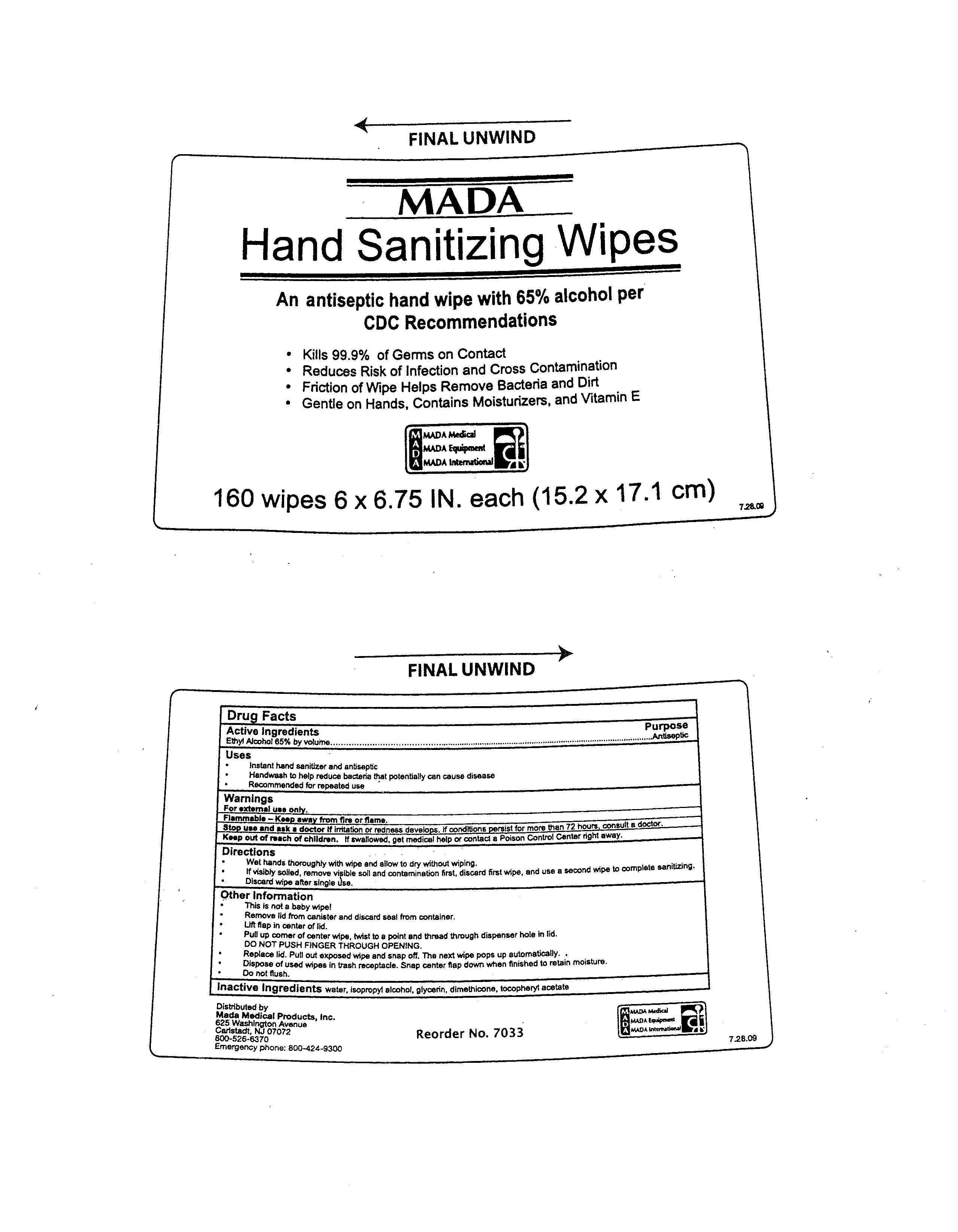
MADA Hand Sanitizing Wipes
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients Purpose
Ethyl Alcohol 65% by volume................................Antiseptic
Uses
Uses
Instant hand sanitizer and antiseptic
Handwash to help reduce bacteria that potentially can cause disease
Recommended for repeated use
Warnings
For external use only.
Flammable - Keep away from fire or flame.
Stop use and ask a doctor if irritation or redness develops.
If conditions persist for more than 72 hours, consult a doctor.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Wet hands thoroughly with wipe and allow to dry without wiping.
If visibly soiled, remove visible soil and contamination first, discard first wipe, and use a second wipe to complete sanitizing.
Discard wipe after single use.
Other Information
This is not a baby wipe!
Remove lid from canister and discard seal from container.
Lift flap in center of lid.
Pull up corner of center wipe, twist to a point and thread through dispenser hole in lid.
DO NOT PUSH FINGER THROUGH OPENING.
Replace lid. Pull out exposed wipe and snap off. The next wipe pops up automatically.
Dispose of used wipes in trash receptacle. Snap center flap down when finished to retain moisture.
Do not flush.
Inactive Ingredients
water, isopropyl alcohol, glycerin, dimethicone, tocopheryl acetate
MADA
Hand Sanitizing Wipes
An antiseptic hand wipe with 65% alcohol per CDC Recommendations
Kills 99.9% of Germs on Contact
Reduces Risk of Infection and Cross Contamination
Friction of Wipes Helps Remove Bacteria and Dirt
Gentle on Hands, Contains Moisturizers, and Vitamin E
160 wipes 6 x 6.75 IN. each (15.2 x 17.1 cm)
MADA Hand Sanitizing WipesAlcohol LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||