Lysol
Lysol No-Touch™ Antibacterial Hand Soap
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Questions? Comments?
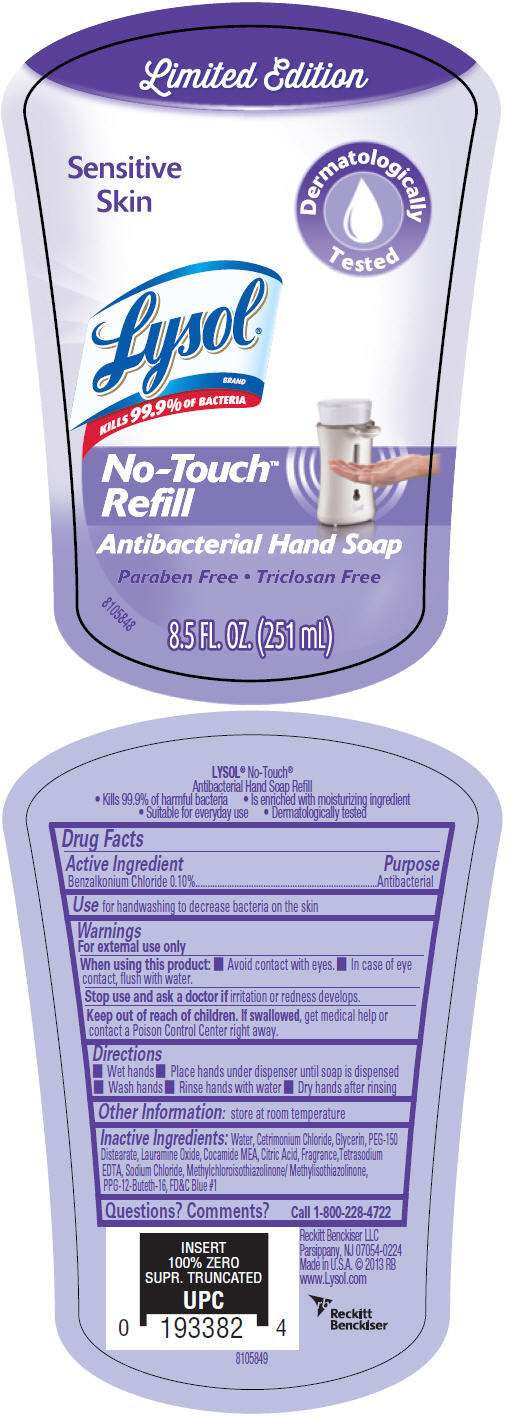
- PRINCIPAL DISPLAY PANEL - 251 mL Bottle Label
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredient
Benzalkonium Chloride 0.10%
Purpose
Antibacterial
Use
for handwashing to decrease bacteria on the skin
Warnings
For external use only
When using this product
- Avoid contact with eyes.
- In case of eye contact, flush with water.
Stop use and ask a doctor if irritation or redness develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Wet hands
- Place hands under dispenser until soap is dispensed
- Wash hands
- Rinse hands with water
- Dry hands after rinsing
Other Information
store at room temperature
Inactive Ingredients
Water, Cetrimonium Chloride, Glycerin, PEG-150 Distearate, Lauramine Oxide, Cocamide MEA, Citric Acid, Fragrance,Tetrasodium EDTA, Sodium Chloride, Methylchloroisothiazolinone/ Methylisothiazolinone, PPG-12-Buteth-16, FD&C Blue #1
Questions? Comments?
Call 1-800-228-4722
Made in U.S.A.
PRINCIPAL DISPLAY PANEL - 251 mL Bottle Label
Limited Edition
Sensitive
Skin
Dermatologically
Tested
Lysol
®
BRAND
KILLS 99.9% OF BACTERIA
No-Touch
™
Refill
Antibacterial Hand Soap
Paraben Free • Triclosan Free
8.5 FL. OZ. (251 mL)
8105848
LysolBenzalkonium Chloride SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||