LYSOL Healthy Touch
Lysol Healthy Touch Antibacterial No-Touch Hand Soap System
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Questions? Comments?
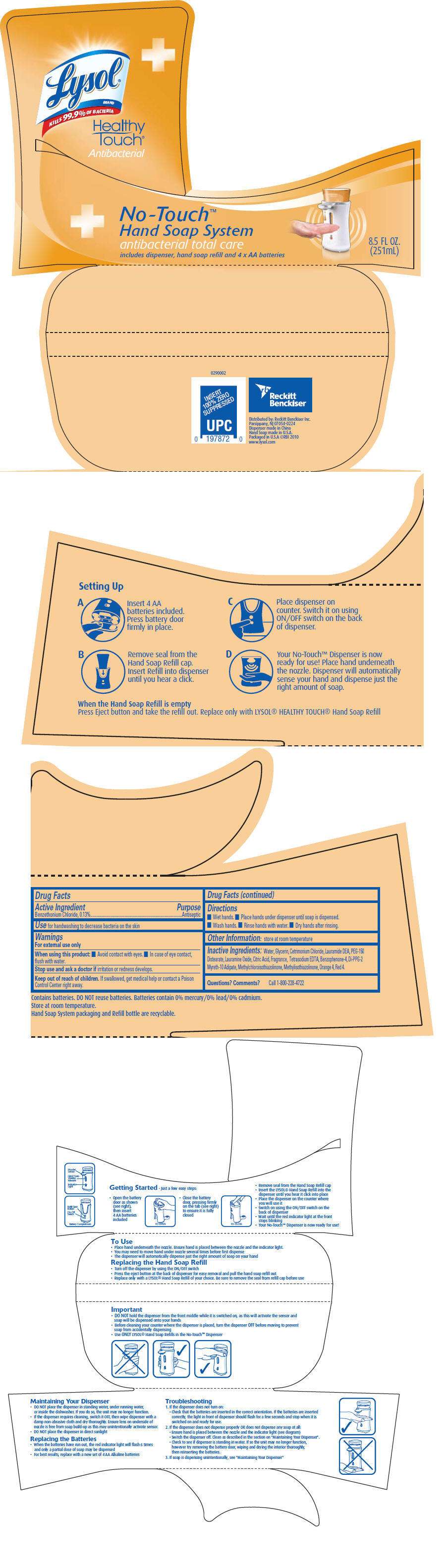
- PRINCIPAL DISPLAY PANEL - 251 mL Bottle Label
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredient
Benzethonium Chloride, 0.13%
Purpose
Antiseptic
Use
for handwashing to decrease bacteria on the skin
Warnings
For external use only
When using this product:
- Avoid contact with eyes.
- In case of eye contact, flush with water.
Stop use and ask a doctor if irritation or redness develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Wet hands.
- Place hands under dispenser until soap is dispensed.
- Wash hands.
- Rinse hands with water.
- Dry hands after rinsing.
Other Information
store at room temperature
Inactive Ingredients
Water, Glycerin, Cetrimonium Chloride, Lauramide DEA, PEG-150 Distearate, Lauramine Oxide, Citric Acid, Fragrance, Tetrasodium EDTA, Benzophenone-4, Di-PPG-2 Myreth-10 Adipate, Methylchloroisothiazolinone, Methylisothiazolinone, Orange 4, Red 4.
Questions? Comments?
Call 1-800-228-4722
Distributed by: Reckitt Benckiser Inc.
Parsippany, NJ 07054-0224
PRINCIPAL DISPLAY PANEL - 251 mL Bottle Label
Lysol
®
BRAND
KILLS 99.9% OF BACTERIA
Healthy
Touch®
Antibacterial
No-Touch™
Hand Soap System
antibacterial total care
includes dispenser, hand soap refill and 4 × AA batteries
8.5 FL OZ.
(251mL)
LYSOL Healthy TouchBenzethonium chloride SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||