Lubas
LUBASBIO CO., LTD
LUBASBIO CO., LTD
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
silicon dioxide
r-aminocapric acid, aluminium chloro hydroxy allantoinate, zeolite, polyedhylene glycol 1500, d-sorbitol solution, glycerine, erythritol, steviol giucoside, xylitol, licorice extract, lactic acid bacteria, sodium carboxymethyl cellulose, methyl parahydroxy benzoate, hydroxylapatite, sodium lauryl sulfate, coolmint flavor, mint flavor, l-menthol, dl-menthol, dionized water
Purpose
for dental care
keep out or reach of the children
Uses
apply 1 to 3 times with desired amount of product onto teeth per day
do not swallow
for dental use only
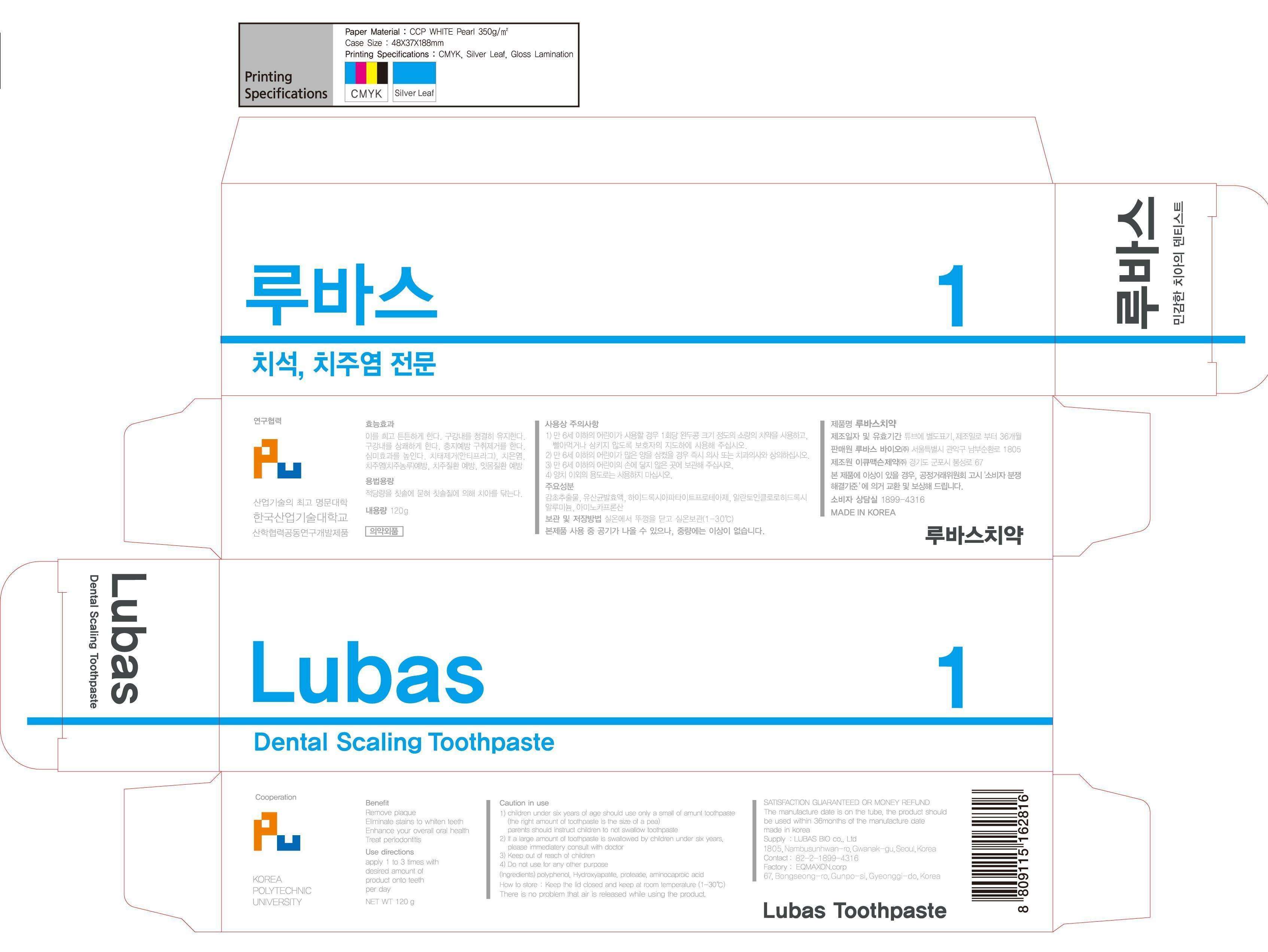
LubasSILICON DIOXIDE PASTE, DENTIFRICE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!