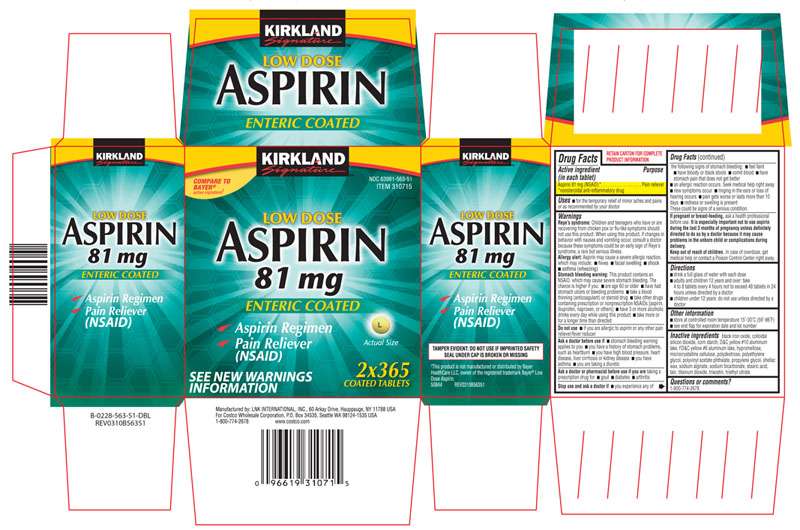
Low Dose Aspirin
COSTCO WHOLESALE CORPORATION
L.N.K. International, Inc.
FULL PRESCRIBING INFORMATION
Aspirin 81 mg (NSAID)*
*nonsteroidal anti-inflammatory drug
Pain Reliever
- for the temporary relief of minor aches and pains or as recommended by your doctor
Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Aspirin may cause a severe allergic reaction, which may include:
- hives
- facial swelling
- shock
- asthma (wheezing)
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription and nonpresctiption NSAID's [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
- if you are allergic to aspirin or any other pain reliever/fever reducer
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis or kidney disease
- you have asthma
- you are taking a diuretic
- taking a prescription drug for
- gout
- diabetes
- arthritis
- you experience any of the following signs of stomach bleeding:
- feel faint
- have bloody or black stools
- vomit blood
- have stomach pain that does not get better
- an allergic reaction occurs. Seek medical help right away.
- new symptoms occur
- ringing in the ears or loss of hearing occurs
- pain gets worse or lasts more than 10 days
- redness or swelling is present
These could be signs of a serious condition.
ask a health professional before use. It is especially important not to use aspirin during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
In case of overdose, get medical help or contact a Poison Control Center right away.
- drink a full glass of water with each dose
- adults and children 12 years and over: take 4 to 8 tablets every 4 hours not to exceed 48 tablets in 24 hours unless directed by a doctor
- children under 12 years: do not use unless directed by a doctor
- store at controlled room temperature 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
black iron oxide, colloidal silicon dioxide, corn starch, D&C yellow #10 aluminum lake, FD&C yellow #6 aluminum lake, hypromellose, microcrystalline cellulose, polydextrose, polyethylene glycol, polyvinyl acetate phthalate, propylene glycol, shellac wax, sodium alginate, sodium bicarbonate, stearic acid, talc, titanium dioxide, triacetin, triethyl citrate
1-800-774-2678
The product packaging shown below represents a sample of that currently in use. Additional packaging may also be available.
KIRKLAND
Signature
COMPARE TO
BEYER®
active ingredient†
LOW DOSE
ASPIRIN
81 mg
ENTERIC COATED
NDC 63981-563-51
ITEM 310715
√ Aspirin Regimen
√ Pain Reliever
(NSAID)
Actual Size
SEE NEW WARNINGS
INFORMATION
2x365
COATED TABLETS
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
50844 REV0310B56351
Manufactured by: LNK INTERNATIONAL,INC.
60 Arkay Drive, Hauppauge, NY 11788 USA
For Costco Wholesale Corporation
P.O. Box 34535, Seattle WA 98124-1535 USA
1-800-774-2678
www.costco.com
Low Dose AspirinAspirin TABLET, COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||