Lovastatin
FULL PRESCRIBING INFORMATION: CONTENTS*
- LOVASTATIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS & USAGE
- LOVASTATIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- LOVASTATIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
LOVASTATIN DESCRIPTION

CLINICAL PHARMACOLOGY
Clinical Studies
PHARMACOKINETICS
PRECAUTIONS, Geriatric Use
WARNINGS, Myopathy/RhabdomyolysisPRECAUTIONS, Drug Interactions
PRECAUTIONS, Drug Interactions
Clinical Studies in Adults
**
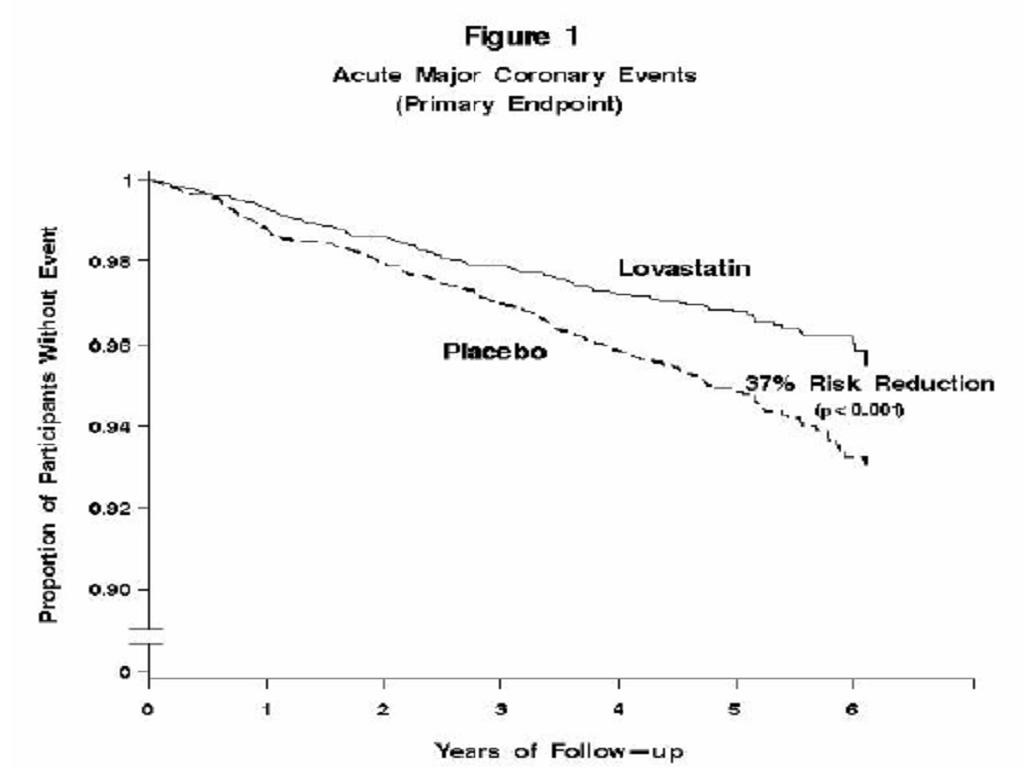
Clinical Studies in Adolescent Patients
**
**
INDICATIONS & USAGE
CLINICAL PHARMACOLOGY, Clinical Studies
*Risk CategoryLDL Goal (mg/dL)LDL Level at Which to Initiate Therapeutic Lifestyle Changes (mg/dL)LDL Level at Which to Consider Drug Therapy (mg/dL)CHD*or CHD risk equivalence (10-year risk >20%)<100(100-129: drug optional)2+ Risk factors (10-year risk<13010-year risk 10-20%:10-year risk<10%:0-1 Risk factor<160(160-189: LDL-lowering drug optional)After the LDL-C goal has been achieved, if the TG is stillmg/dL, non-HDL-C (total-C minus HDLC) becomes a secondary target of therapy. Non-HDL-C goals are set 30 mg/dL higher than LDL-C goals for each risk category.
At the time of hospitalization for an acute coronary event, consideration can be given to initiating drug therapy at discharge if the LDL-C ismg/dL (see NCEP Guidelines above).
Since the goal of treatment is to lower LDL-C, the NCEP recommends that LDL-C levels be used to initiate and assess treatment response. Only if LDL-C levels are not available, should the total-C be used to monitor therapy.
Although lovastatin tablets may be useful to reduce elevated LDL-C levels in patients with combined hypercholesterolemia and hypertriglyceridemia where hypercholesterolemia is the major abnormality (Type IIb hyperlipoproteinemia), it has not been studied in conditions where the major abnormality is elevation of chylomicrons, VLDL or IDL (i.e., hyperlipoproteinemia types I, III, IV, or V).***
The NCEP classification of cholesterol levels in pediatric patients with a familial history of hypercholesterolemia or premature cardiovascular disease is summarized below:
CategoryTotal-C (mg/dL)LDL-C (mg/dL)Acceptable<170<110Borderline170-199110-129HighChildren treated with lovastatin in adolescence should be re-evaluated in adulthood and appropriate changes made to their cholesterol-lowering regimen to achieve adult goals for LDL-C.
LOVASTATIN CONTRAINDICATIONS
WARNINGS
PRECAUTIONS, PregnancyNursing MothersPRECAUTIONS, Pregnancy
WARNINGS
Myopathy/RhabdomyolysisCLINICAL PHARMACOLOGY, PharmacokineticsPRECAUTIONS, Drug InteractionsDOSAGE AND ADMINISTRATION).
Table VI. Drug Interactions Associated with Increased Risk of Myopathy/ Rhabdomyolysis
Interacting AgentsPrescribing RecommendationsItraconazole Ketoconazole Erythromycin Clarithromycin Telithromycin HIV protease inhibitors NefazodoneAvoid lovastatinGemfibrozil Other fibrates Lipid-lowering doses (g/day) of niacin Cyclosporine DanozolDo not exceed 20 mg lovastatin dailyAmiodarone VerapamilDo not exceed 40 mg lovastatin dailyGrapefruit juiceAvoid large quantities of grapefruit juice (>1 quart daily)
Liver Dysfunction
Persistent increases (to more than 3 times the upper limit of normal) in serum transaminases occurred in 1.9% of adult patients who received lovastatin for at least one year in early clinical trials (seeADVERSE REACTIONS). When the drug was interrupted or discontinued in these patients, the transaminase levels usually fell slowly to pretreatment levels. The increases usually appeared 3 to 12 months after the start of therapy with lovastatin, and were not associated with jaundice or other clinical signs or symptoms. There was no evidence of hypersensitivity. In the EXCEL study (seeCLINICAL PHARMACOLOGY, Clinical Studies), the incidence of persistent increases in serum transaminases over 48 weeks was 0.1% for placebo, 0.1% at 20 mg/day, 0.9% at 40 mg/day, and 1.5% at 80 mg/day in patients on lovastatin. However, in post-marketing experience with lovastatin, symptomatic liver disease has been reported rarely at all dosages (seeADVERSE REACTIONS).
In AFCAPS/TexCAPS, the number of participants with consecutive elevations of either alanine aminotransferase (ALT) or aspartate aminotransferase (AST) (> 3 times the upper limit of normal), over a median of 5.1 years of follow-up, was not significantly different between the lovastatin and placebo groups (18 [0.6%] vs. 11 [0.3%]). The starting dose of lovastatin was 20 mg/day; 50% of the lovastatin treated participants were titrated to 40 mg/day at Week 18. Of the 18 participants on lovastatin with consecutive elevations of either ALT or AST, 11 (0.7%) elevations occurred in participants taking 20 mg/day, while 7 (0.4%) elevations occurred in participants titrated to 40 mg/day. Elevated transaminases resulted in discontinuation of 6 (0.2%) participants from therapy in the lovastatin group (n=3,304) and 4 (0.1%) in the placebo group (n=3,301).
It is recommended that liver function tests be performed prior to initiation of therapy in patients with a history of liver disease, or when otherwise clinically indicated. It is recommended that liver function tests be performed in all patients prior to use of 40 mg or more daily and thereafter when clinically indicated. Patients who develop increased transaminase levels should be monitored with a second liver function evaluation to confirm the finding and be followed thereafter with frequent liver function tests until the abnormality(ies) returns to normal. Should an increase in AST or ALT of three times the upper limit of normal or greater persist, withdrawal of therapy with lovastatin is recommended.
The drug should be used with caution in patients who consume substantial quantities of alcohol and/or have a past history of liver disease. Active liver disease or unexplained transaminase elevations are contraindications to the use of lovastatin.
As with other lipid-lowering agents, moderate (less than three times the upper limit of normal) elevations of serum transaminases have been reported following therapy with lovastatin (seeADVERSE REACTIONS. These changes appeared soon after initiation of therapy with lovastatin, were often transient, were not accompanied by any symptoms and interruption of treatment was not required.
PRECAUTIONS
GeneralWARNINGSADVERSE REACTIONS
ADVERSE REACTIONS
INFORMATION FOR PATIENTS
WARNINGS, Myopathy/RhabdomyolysisDRUG INTERACTIONS
WARNINGS, Myopathy/RhabdomyolysisCLINICAL PHARMACOLOGY, Pharmacokinetics
WARNINGS, Myopathy/Rhabdomyolysis
Other drug interactions
WARNINGS, Myopathy/RhabdomyolysisCLINICAL PHARMACOLOGY, Pharmacokinetics).
Amiodarone or Verapamil: The risk of myopathy/rhabdomyolysis is increased when either amiodarone or verapamil is used concomitantly with a closely related member of the HMG-CoA reductase inhibitor class (seeWARNINGS, Myopathy/Rhabdomyolysis).
Coumarin Anticoagulants: In a small clinical trial in which lovastatin was administered to warfarin treated patients, no effect on prothrombin time was detected. However, another HMG-CoA reductase inhibitor has been found to produce a less than two-second increase in prothrombin time in healthy volunteers receiving low doses of warfarin. Also, bleeding and/or increased prothrombin time have been reported in a few patients taking coumarin anticoagulants concomitantly with lovastatin. It is recommended that in patients taking anticoagulants, prothrombin time be determined before starting lovastatin and frequently enough during early therapy to insure that no significant alteration of prothrombin time occurs. Once a stable prothrombin time has been documented, prothrombin times can be monitored at the intervals usually recommended for patients on coumarin anticoagulants. If the dose of lovastatin is changed, the same procedure should be repeated. Lovastatin therapy has not been associated with bleeding or with changes in prothrombin time in patients not taking anticoagulants.
Propranolol: In normal volunteers, there was no clinically significant pharmacokinetic or pharmacodynamic interaction with concomitant administration of single doses of lovastatin and propranolol.
Digoxin: In patients with hypercholesterolemia, concomitant administration of lovastatin and digoxin resulted in no effect on digoxin plasma concentrations.
Oral Hypoglycemic Agents: In pharmacokinetic studies of lovastatin in hypercholesterolemic noninsulin dependent diabetic patients, there was no drug interaction with glipizide or with chlorpropamide (seeCLINICAL PHARMACOLOGY, Clinical Studies).
Endocrine Function
HMG-CoA reductase inhibitors interfere with cholesterol synthesis and as such might theoretically blunt adrenal and/or gonadal steroid production. Results of clinical trials with drugs in this class have been inconsistent with regard to drug effects on basal and reserve steroid levels. However, clinical studies have shown that lovastatin does not reduce basal plasma cortisol concentration or impair adrenal reserve, and does not reduce basal plasma testosterone concentration. Another HMG-CoA reductase inhibitor has been shown to reduce the plasma testosterone response to HCG. In the same study, the mean testosterone response to HCG was slightly but not significantly reduced after treatment with lovastatin 40 mg daily for 16 weeks in 21 men. The effects of HMG-CoA reductase inhibitors on male fertility have not been studied in adequate numbers of male patients. The effects, if any, on the pituitary-gonadal axis in pre-menopausal women are unknown. Patients treated with lovastatin who develop clinical evidence of endocrine dysfunction should be evaluated appropriately. Caution should also be exercised if an HMG-CoA reductase inhibitor or other agent used to lower cholesterol levels is administered to patients also receiving other drugs (e.g., ketoconazole, spironolactone, cimetidine) that may decrease the levels or activity of endogenous steroid hormones.
CNS Toxicity
Lovastatin produced optic nerve degeneration (Wallerian degeneration of retinogeniculate fibers) in clinically normal dogs in a dose-dependent fashion starting at 60 mg/kg/day, a dose that produced mean plasma drug levels about 30 times higher than the mean drug level in humans taking the highest recommended dose (as measured by total enzyme inhibitory activity). Vestibulocochlear Wallerian-like degeneration and retinal ganglion cell chromatolysis were also seen in dogs treated for 14 weeks at 180 mg/kg/day, a dose which resulted in a mean plasma drug level (Cmax) similar to that seen with the 60 mg/kg/day dose.
CNS vascular lesions, characterized by perivascular hemorrhage and edema, mononuclear cell infiltration of perivascular spaces, perivascular fibrin deposits and necrosis of small vessels, were seen in dogs treated with lovastatin at a dose of 180 mg/kg/day, a dose which produced plasma drug levels (Cmax) which were about 30 times higher than the mean values in humans taking 80 mg/day.
Similar optic nerve and CNS vascular lesions have been observed with other drugs of this class.
Cataracts were seen in dogs treated for 11 and 28 weeks at 180 mg/kg/day and 1 year at 60 mg/kg/day.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Pregnancy Category XCONTRAINDICATIONS
CONTRAINDICATIONS
NURSING MOTHERS
CONTRAINDICATIONSPEDIATRIC USE
CLINICAL PHARMACOLOGY, Clinical Studies in Adolescent PatientsADVERSE REACTIONS, Adolescent PatientsDOSAGE AND ADMINISTRATION, Adolescent Patients (10-17 years of age) with Heterozygous Familial Hypercholesterolemia.Adolescent females should be counseled on appropriate contraceptive methods while on lovastatin therapy (seeCONTRAINDICATIONSandPRECAUTIONS, Pregnancy). Lovastatin has not been studied in pre-pubertal patients or patients younger than 10 years of age.GERIATRIC USE
CLINICAL PHARMACOLOGYLOVASTATIN ADVERSE REACTIONS
Phase III Clinical Studies
Expanded Clinical Evaluation of Lovastatin [EXCEL] Study
WARNINGS, Liver DysfunctionWARNINGS, Myopathy/Rhabdomyolysis
Expanded Clinical Evaluation of Lovastatin (EXCEL) Study
CLINICAL PHARMACOLOGY, Clinical Studies
CLINICAL PHARMACOLOGY, Clinical StudiesADVERSE REACTIONS, Expanded Clinical Evaluation of Lovastatin [EXCEL] Study
WARNINGS, Myopathy/Rhabdomyolysis
Adolescent Patients (ages 10-17 years)
CLINICAL PHARMACOLOGY, Clinical Studies in Adolescent PatientsPRECAUTIONS, Pediatric Use
OVERDOSAGE
DOSAGE & ADMINISTRATION
Adult Patients
CLINICAL PHARMACOLOGYINDICATIONS AND USAGE
Dosage in Patients taking Cyclosporine or Danazol
WARNINGS, Myopathy/Rhabdomyolysis
Dosage in Patients taking Amiodarone or Verapamil
WARNINGS, Myopathy/RhabdomyolysisPRECAUTIONS, Drug InteractionsOther drug interactions
Adolescent Patients (10-17 years of age) with Heterozygous Familial Hypercholesterolemia
CLINICAL PHARMACOLOGYINDICATIONS AND USAGE
Concomitant Lipid-Lowering Therapy
WARNINGS, Myopathy/RhabdomyolysisPRECAUTIONS, Drug Interactions
Dosage in Patients with Renal Insufficiency
CLINICAL PHARMACOLOGYWARNINGS, Myopathy/Rhabdomyolysis
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

LovastatinLovastatin TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!