Losartan Potassium
Endo Pharmaceuticals Inc. DBA Endo Generic Products
Alembic Pharmaceuticals Limited
Losartan Potassium Tablets USP R only
FULL PRESCRIBING INFORMATION: CONTENTS*
- LOSARTAN POTASSIUM DESCRIPTION
- CLINICAL PHARMACOLOGY
- LOSARTAN POTASSIUM INDICATIONS AND USAGE
- LOSARTAN POTASSIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LOSARTAN POTASSIUM ADVERSE REACTIONS
- OVERDOSAGE
- LOSARTAN POTASSIUM DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- SUPPLEMENTAL PATIENT MATERIAL
FULL PRESCRIBING INFORMATION
|
USE IN PREGNANCY When used in pregnancy during the second and third trimesters, drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus. When pregnancy is detected, losartan potassium tablets should be discontinued as soon as possible. See WARNINGS, Fetal/Neonatal Morbidity and Mortality. |
LOSARTAN POTASSIUM DESCRIPTION
1
22226

CLINICAL PHARMACOLOGY
Mechanism of Action
1e.g.,2112In vitro11
Pharmacokinetics
General
14In vitro
max
1414
Special Populations
Pediatric
| |
Adults given 50 mg once daily for 7 days N = 12 |
Age 6-16 given 0.7 mg/kg once daily for 7 days N = 25 |
||
| |
Parent |
Active Metabolite |
Parent |
Active Metabolite |
| AUC0-24
a (ng•h/mL) |
442 ± 173 |
1685 ± 452 |
368 ± 169 |
1866 ± 1076 |
| Cmax (ng/mL) a
|
224 ± 82 |
212 ± 73 |
141 ± 88 |
222 ± 127 |
| T1/2 (h) b
|
2.1 ± 0.70 |
7.4 ± 2.4 |
2.3 ± 0.8 |
5.6 ± 1.2 |
| Tpeak (h) c
|
0.9 |
3.5 |
2.0 |
4.1 |
| CLren (mL/min) a
|
56 ± 23 |
20 ± 3 |
53 ± 33 |
17 ± 8 |
a
b
c
DOSAGE AND ADMINISTRATIONPreparation of Suspension)
Geriatric and GenderDOSAGE AND ADMINISTRATION
RacePRECAUTIONSRaceCLINICAL PHARMACOLOGYPharmacodynamics and Clinical Effects, Reduction in the Risk of Stroke, Race
Renal InsufficiencyWARNINGSHypotension - Volume-Depleted PatientsDOSAGE AND ADMINISTRATION
Hepatic InsufficiencyDOSAGE AND ADMINISTRATION
Drug Interactions
Pharmacodynamics and Clinical Effects
Adult Hypertension
Pediatric Hypertension
DOSAGE AND ADMINISTRATIONPreparation of Suspensionth
Reduction in the Risk of Stroke: e.g.,
e.g.,
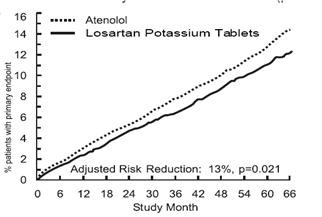
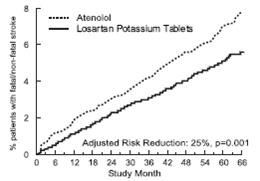
Table 2 Incidence of Primary Endpoint Events
| |
Losartan potassium tablets |
Atenolol |
Risk Reduction† |
95% CI |
p-Value |
||
| |
N (%) |
Rate* |
N (%) |
Rate* |
|
|
|
| Primary Composite Endpoint |
508 (11) |
23.8 |
588 (13) |
27.9 |
13% |
2% to 23% |
0.021 |
| Components of Primary Composite Endpoint (as a first event) |
|
||||||
| Stroke (nonfatal ‡) |
209 (5) |
|
286 (6) |
|
|
|
|
| Myocardial infarction (nonfatal ‡) |
174 (4) |
|
168 (4) |
|
|
|
|
| Cardiovascular mortality |
125 (3) |
|
134 (3) |
|
|
|
|
| Secondary Endpoints (any time in study) |
|
||||||
| Stroke (fatal/nonfatal) |
232 (5) |
10.8 |
309 (7) |
14.5 |
25% |
11% to 37% |
0.001 |
| Myocardial infarction (fatal/nonfatal) |
198 (4) |
9.2 |
188 (4) |
8.7 |
-7% |
-13% to 12% |
0.491 |
| Cardiovascular mortality |
204 (4) |
9.2 |
234 (5) |
10.6 |
11% |
-7% to 27% |
0.206 |
| Due to CHD |
125 (3) |
5.6 |
124 (3) |
5.6 |
-3% |
-32% to 20% |
0.839 |
| Due to Stroke |
40 (1) |
1.8 |
62 (1) |
2.8 |
35% |
4% to 67% |
0.032 |
| Other §
|
39 (1) |
1.8 |
48 (1) |
2.2 |
16% |
-28% to 45% |
0.411 |
| * Rate per 1000 patient-years of follow-up |
|||||||
| † Adjusted for baseline Framingham risk score and level of electrocardiographic left ventricular hypertrophy |
|||||||
| ‡ First report of an event, in some cases the patient died subsequently to the event reported |
|||||||
| § Death due to heart failure, non-coronary vascular disease, pulmonary embolism, or a cardiovascular cause other than stroke or coronary heart disease |
|||||||
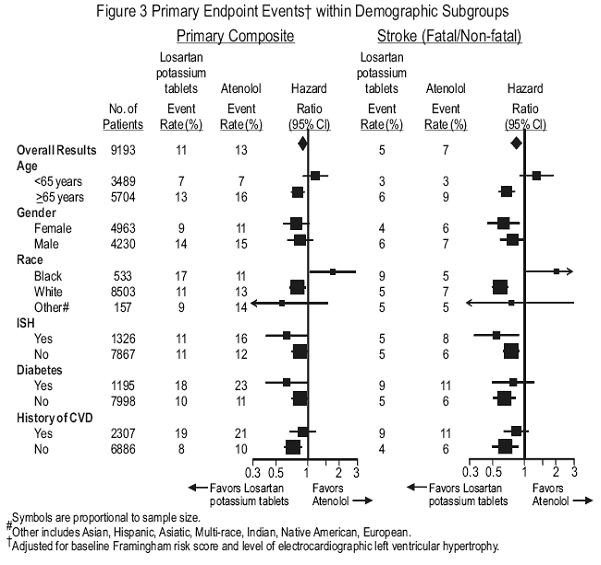
Race:
Nephropathy in Type 2 Diabetic Patients:<>
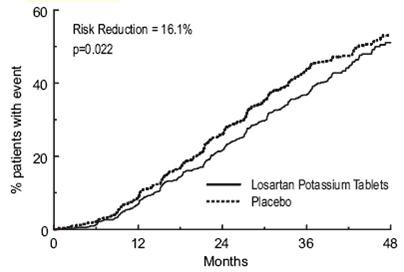
| |
Incidence |
Risk Reduction |
95% C.I. |
p-Value |
|
| |
Losartan |
Placebo |
|
|
|
| Primary Composite Endpoint |
43.5% |
47.1% |
16.1% |
2.3% to 27.9% |
0.022 |
| Doubling of Serum Creatinine, ESRD and Death Occurring as a First Event |
|||||
| Doubling of Serum Creatinine |
21.6% |
26.0% |
|
|
|
| ESRD |
8.5% |
8.5% |
|
|
|
| Death |
13.4% |
12.6% |
|
|
|
| Overall Incidence of Doubling of Serum Creatinine, ESRD and Death |
|||||
| Doubling of Serum Creatinine |
21.6% |
26.0% |
25.3% |
7.8% to 39.4% |
0.006 |
| ESRD |
19.6% |
25.5% |
28.6% |
11.5% to 42.4% |
0.002 |
| Death |
21.0% |
20.3% |
-1.7% |
-26.9% to 18.6% |
0.884 |
| |
No. of Patients |
Primary Composite Endpoint |
ESRD |
||||
| Losartan potassium tablets Event Rate % |
Placebo Event Rate % |
Hazard Ratio (95% CI) |
Losartan potassium tablets Event Rate % |
Placebo Event Rate % |
Hazard Ratio (95% CI) |
||
| Overall Results |
1513 |
43.5 |
47.1 |
0.839 (0.721, 0.977) |
19.6 |
25.5 |
0.714 (0.576, 0.885) |
| Age |
|
|
|
|
|
|
|
| <65 years |
1005 |
44.1 |
49.0 |
0.784 (0.653, 0.941) |
21.1 |
28.5 |
0.670 (0.521, 0.863) |
| ≥65 years |
508 |
42.3 |
43.5 |
0.978 (0.749, 1.277) |
16.5 |
19.6 |
0.847 (0.560, 1.281) |
| Gender |
|
|
|
|
|
|
|
| Female |
557 |
47.8 |
54.1 |
0.762 (0.603, 0.962) |
22.8 |
32.8 |
0.601 (0.436, 0.828) |
| Male |
956 |
40.9 |
43.3 |
0.892 (0.733, 1.085) |
17.5 |
21.5 |
0.809 (0.605, 1.081) |
| Race |
|
|
|
|
|
|
|
| Asian |
252 |
41.9 |
54.8 |
0.655 (0.453, 0.947) |
18.8 |
27.4 |
0.625 (0.367, 1.066) |
| Black |
230 |
40.0 |
39.0 |
0.983 (0.647, 1.495) |
17.6 |
21.0 |
0.831 (0.456, 1.516) |
| Hispanic |
277 |
55.0 |
54.0 |
1.003 (0.728, 1.380) |
30.0 |
28.5 |
1.024 (0.661, 1.586) |
| White |
735 |
40.5 |
43.2 |
0.809 (0.645, 1.013) |
16.2 |
23.9 |
0.596 (0.427, 0.831) |
LOSARTAN POTASSIUM INDICATIONS AND USAGE
Hypertension
Hypertensive Patients with Left Ventricular Hypertrophy
PRECAUTIONS, RaceCLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, Reduction in the Risk of Stroke, Race
Nephropathy in Type 2 Diabetic Patients
CLINICAL PHARMACOLOGYPharmacodynamics and Clinical Effects
LOSARTAN POTASSIUM CONTRAINDICATIONS
Losartan potassium tablets are contraindicated in patients who are hypersensitive to any component of this product.
WARNINGS
Fetal/Neonatal Morbidity and Mortality
in utero
2
Hypotension - Volume-Depleted Patients
e.g.,DOSAGE AND ADMINISTRATION).
PRECAUTIONS
General
Hypersensitivity: ADVERSE REACTIONS,Post-Marketing Experience.
Impaired Hepatic Function
DOSAGE AND ADMINISTRATIONCLINICAL PHARMACOLOGY,Pharmacokinetics
Impaired Renal Function
e.g.,
Electrolyte Imbalance
ADVERSE REACTIONS
Information for Patients
Pregnancy:
Potassium Supplements:PRECAUTIONS, Drug Interactions
Drug Interactions
CLINICAL PHARMACOLOGY, Drug Interactions
e.g.,
Lithium:
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors):
Carcinogenesis, Mutagenesis, Impairment of Fertility
in vitroin vitroin vivoin vitroin vitro
Pregnancy
Pregnancy Categories C (first trimester) and D (second and third trimesters). See WARNINGS, Fetal/Neonatal Morbidity and Mortality.
Nursing Mothers
It is not known whether losartan is excreted in human milk, but significant levels of losartan and its active metabolite were shown to be present in rat milk. Because of the potential for adverse effects on the nursing infant, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Antihypertensive effects of losartan potassium tablets have been established in hypertensive pediatric patients aged 6 to 16 years. There are no data on the effect of losartan potassium tablets on blood pressure in pediatric patients under the age of 6 or in pediatric patients with glomerular filtration rate <30 mL/min/1.73 m2 (see CLINICAL PHARMACOLOGY, Pharmacokinetics, Special Populations and Pharmacodynamics and Clinical Effects and DOSAGE AND ADMINISTRATION).
Geriatric Use
Race
CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, Reduction in the Risk of Stroke).
LOSARTAN POTASSIUM ADVERSE REACTIONS
Hypertension
| |
Losartan (n = 1075) Incidence % |
Placebo (n = 334) Incidence % |
|
Musculoskeletal
Cramp, muscle Pain, back Pain, leg |
1 2 1 |
0 1 0 |
|
Nervous
System/Psychiatric
Dizziness |
3 |
2 |
|
Respiratory
Congestion, nasal Infection, upper respiratory Sinusitis |
2 8 1 |
1 7 0 |
Body as a Whole:
Cardiovascular
Digestive:
Hematologic:
Metabolic:
Musculoskeletal:
Nervous System/Psychiatric:
Respiratory:
Skin:
Special Senses:
Urogenital:
| Study 1 † |
HCTZ |
Losartan |
Lisinopril |
| Cough |
25% |
17% |
69% |
| |
|
|
|
| Study 2 †† |
Placebo |
Losartan |
Lisinopril |
| Cough |
35% |
29% |
62% |
† Demographics = (89% caucasian, 64% female)
†† Demographics = (90% caucasian, 51% female)
Pediatric Patients:
Hypertensive Patients with Left Ventricular Hypertrophy
Nephropathy in Type 2 Diabetic Patients
>
Post-Marketing Experience
|
|
Losartan and Conventional Antihypertensive Therapy Incidence % (n=751) |
Placebo and Conventional Antihypertensive Therapy Incidence % (n=762) |
|
Body as a Whole
|
|
|
| Asthenia/Fatigue |
14 |
10 |
| Chest Pain
|
12 |
8 |
| Fever
|
4 |
3 |
| Infection
|
5 |
4 |
| Influenza-like disease
|
10 |
9 |
| Trauma
|
4 |
3 |
|
Cardiovascular
|
|
|
| Hypotension
|
7 |
3 |
| Orthostatic hypotension
|
4 |
1 |
|
Digestive
|
|
|
| Diarrhea
|
15 |
10 |
| Dyspepsia
|
4 |
3 |
| Gastritis
|
5 |
4 |
|
Endocrine
|
|
|
| Diabetic neuropathy
|
4 |
3 |
| Diabetic vascular disease
|
10 |
9 |
|
Eyes, Ears, Nose and Throat
|
|
|
| Cataract
|
7 |
5 |
| Sinusitis
|
6 |
5 |
|
Hemic
|
|
|
| Anemia
|
14 |
11 |
|
Metabolic and Nutrition
|
|
|
| Hyperkalemia
|
7 |
3 |
| Hypoglycemia
|
14 |
10 |
| Weight gain
|
4 |
3 |
|
Musculoskeletal
|
|
|
| Back pain
|
12 |
10 |
| Leg pain
|
5 |
4 |
| Knee pain |
5 |
4 |
| Muscular weakness
|
7 |
4 |
|
Nervous System
|
|
|
| Hypesthesia |
5 |
4 |
|
Respiratory
|
|
|
| Bronchitis |
10 |
9 |
| Cough |
11 |
10 |
|
Skin
|
|
|
| Cellulitis |
7 |
6 |
|
Urogenital
|
|
|
| Urinary tract infection
|
16 |
13 |
Post-Marketing Experience
Digestive:
General Disorders and Administration Site Conditions:
Hemic:
Hypersensitivity:
Metabolic and Nutrition:
Musculoskeletal:
Nervous system disorders:
Respiratory:
Skin:
Laboratory Test Findings
Creatinine, Blood Urea Nitrogen:PRECAUTIONS, Impaired Renal Function
Hemoglobin and Hematocrit:
Liver Function Tests:
OVERDOSAGE
2
LOSARTAN POTASSIUM DOSAGE AND ADMINISTRATION
Adult Hypertensive Patients
e.g.,WARNINGS, Hypotension - Volume-Depleted PatientsPRECAUTIONS, General
CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, Hypertension
CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, Hypertension
Pediatric Hypertensive Patients ≥ 6 years of age
Preparation of SuspensionCLINICAL PHARMACOLOGY, Pharmacokinetics, Special Populations Pharmacodynamics and Clinical Effects,WARNINGS, Hypotension - Volume-Depleted Patients
2CLINICAL PHARMACOLOGY, Pharmacokinetics, Special Populations, Pharmacodynamics and Clinical Effects,PRECAUTIONS
Preparation of Suspension (for 200 mL of a 2.5 mg/mL suspension)
****
Hypertensive Patients with Left Ventricular Hypertrophy
CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, Reduction in the Risk of Stroke
Nephropathy in Type 2 Diabetic Patients
CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical EffectsNephropathy in Type 2 Diabetic Patientse.g.,
HOW SUPPLIED
60951-185
Storage:
** Trademark of Paddock Laboratories, Inc.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Losartan Potassium Tablets USP 25 mg (90 Tablets in HDPE bottle)
Each film coated tablet contains: Losartan Potassium USP 25 mg
NDC Code: 60951-185-92

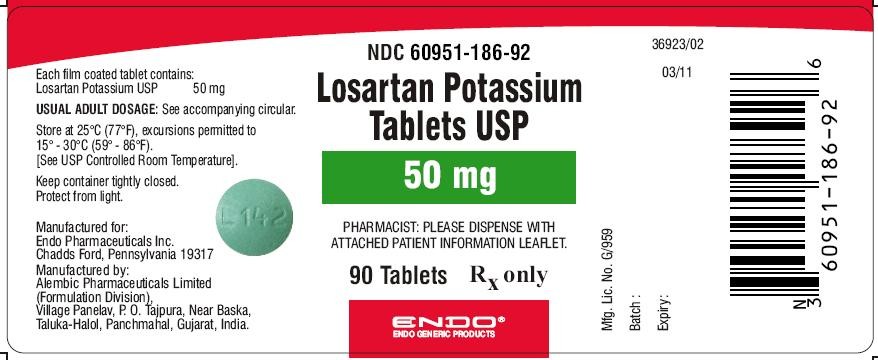
Losartan Potassium Tablets USP 50 mg (90 Tablets in HDPE bottle)
Each film coated tablet contains: Losartan Potassium USP 50 mg
NDC Code: 60951-186-92
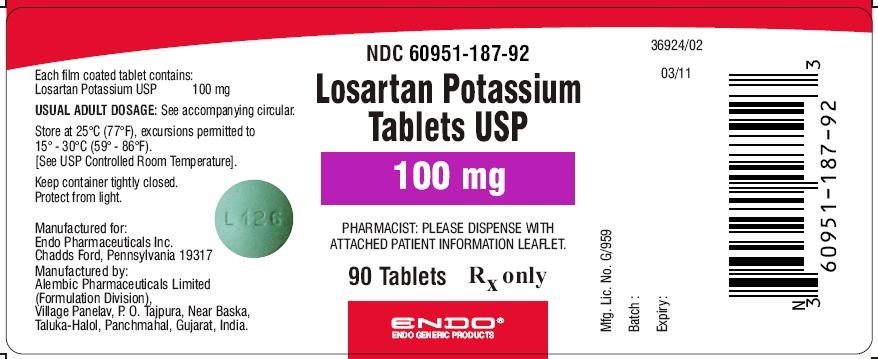
Losartan Potassium Tablets USP 100 mg (90 Tablets in HDPE bottle)
Each film coated tablet contains: Losartan Potassium USP 100 mg
NDC Code: 60951-187-92
SUPPLEMENTAL PATIENT MATERIAL
Patient Package Insert
Patient Information
Losartan Potassium Tablets USP
25 mg, 50 mg, 100 mg
Rx only
What is the most important information I should know about losartan potassium tablets?
Do not take losartan potassium tablets if you are pregnant or plan to become pregnant. Losartan potassium tablets can harm your unborn baby causing injury and even death. Stop taking losartan potassium tablets if you become pregnant and call your doctor right away.
What is losartan potassium tablet?
- alone or with other blood pressure medicines to lower high blood pressure (hypertension).
- to lower the chance of stroke in patients with high blood pressure and a heart problem called left ventricular hypertrophy. Losartan potassium tablets may not help Black patients with this problem.
- to slow the worsening of diabetic kidney disease (nephropathy) in patients with type 2 diabetes who have or had high blood pressure.
High Blood Pressure (hypertension).
Left Ventricular Hypertrophy (LVH)
Type 2 Diabetes with Nephropathy.
Who should not take losartan potassium tablets?
Do not take losartan potassium tablets if you are allergic to any of the ingredients in losartan potassium tablets.
What should I tell my doctor before taking losartan potassium tablets?
- are pregnant or planning to become pregnant. See “What is the most important information I should know about losartan potassium tablets?”·
- are breast-feeding. It is not known if losartan potassium tablets pass into your breast milk. You should choose either to take losartan potassium tablets or breast-feed, but not both.
- are vomiting a lot or having a lot of diarrhea
- have liver problems
- have kidney problems
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
- potassium supplements
- salt substitutes containing potassium
- water pills (diuretics)
- medicines used to treat pain and arthritis, called non-steroidal anti-inflammatory drugs (NSAIDs), including COX-2 inhibitors.
How should I take losartan potassium tablets?
- Take losartan potassium tablets exactly as prescribed by your doctor. Your doctor may change your dose if needed.
- Losartan potassium tablets can be taken with or without food.
- If you miss a dose, take it as soon as you remember. If it is close to your next dose, do not take the missed dose. Just take the next dose at your regular time.
- If you take too much losartan potassium tablets, call your doctor or Poison Control Center, or go to the nearest hospital emergency room right away.
What are the possible side effects of losartan potassium tablets?
- Injury or death of unborn babies. See “What is the most important information I should know about losartan potassium tablets?”
- Allergic reaction. Symptoms of an allergic reaction are swelling of the face, lips, throat or tongue. Get emergency medical help right away and stop taking losartan potassium tablets.
- Low blood pressure (hypotension). Low blood pressure may cause you to feel faint or dizzy. Lie down if you feel faint or dizzy. Call your doctor right away.
- For people who already have kidney problems, you may see a worsening in how well your kidneys work. Call your doctor if you get swelling in your feet, ankles, or hands, or unexplained weight gain.
The most common side effects of losartan potassium tablets in people with high blood pressure are:
- “colds” (upper respiratory infection)
- dizziness
- stuffy nose
- back pain
- diarrhea
- tiredness
- low blood sugar
- chest pain
- high blood potassium
- low blood pressure
not
How do I store losartan potassium tablets?
- Store losartan potassium tablets at 25°C (77°F); excursion permitted to 15 - 30°C (59 - 86°F).
- Keep losartan potassium tablets in a tightly closed container that protects the medicine from light.
- Keep losartan potassium tablets and all medicines out of the reach of children.
General information about losartan potassium tablets
What are the ingredients in losartan potassium tablets?
Active ingredients:
Inactive ingredients:
Losartan PotassiumLosartan Potassium TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Losartan PotassiumLosartan Potassium TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Losartan PotassiumLosartan Potassium TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||