Loratadine
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each tablet)
- Purpose
- Loratadine Uses
- Warnings
- Directions (24 Hour Relief)
- Loratadine Other information
- Inactive ingredients
- Questions or comments?
FULL PRESCRIBING INFORMATION
Original Prescription Strength
Non-Drowsy
Indoor and Outdoor Allergies
TAMPER EVIDENT: DO NOT USE IF BLISTER UNITS ARE TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
Active ingredient (in each tablet)
Loratadine USP, 10 mg
Purpose
Antihistamine
Loratadine Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
Warnings
Do not use
if you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions (24 Hour Relief)
| adults and children 6 years and over |
1 tablet daily; not more than 1 tablet in 24 hours |
| children under 6 years of age | ask a doctor |
|
consumers with liver |
ask a doctor |
Loratadine Other information
- TAMPER EVIDENT: DO NOTUSE IF BLISTER UNITS ARE TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
- store between 20° to 25°C (68° to 77°F)
- protect from excessive moisture
Inactive ingredients
Anhydrous lactose, colloidal silicon dioxide, corn starch, hypromellose, magnesium stearate, microcrystalline cellulose, povidone and sodium lauryl sulfate.
Questions or comments?
1-800-848-0462
- Serious side effects associated with use of this product may be reported to this number.
Manufactured by:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Distributed by:
UDL Laboratories, Inc.
Rockford, IL 61103
S-10314 R1
7/11
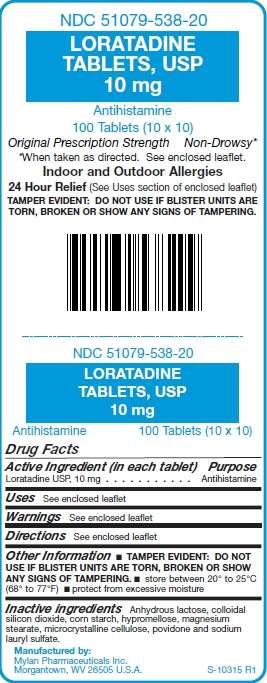
NDC 51079-538-20
LORATADINE
TABLETS, USP
10 mg
Antihistamine
100 Tablets (10 x 10)
Original Prescription Strength Non-Drowsy*
*When taken as directed. See enclosed leaflet.
Indoor and Outdoor Allergies
24 Hour Relief (See Uses section of enclosed leaflet)
TAMPER EVIDENT: DO NOT USE IF
BLISTER UNITS ARE TORN, BROKEN OR
SHOW ANY SIGNS OF TAMPERING
Drug Facts
Active Ingredient (in each tablet) Purpose
Loratadine USP, 10 mg . . . . . . . . . . . Antihistamine
Uses See enclosed leaflet
Warnings See enclosed leaflet
Directions See enclosed leaflet
Other Information
- TAMPER EVIDENT: DO NOT USE IF BLISTER UNITS ARE TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
- store between 20° to 25°C (68° to 77°F) ̣̣
- protect from excessive moisture
Inactive ingredients
Anhydrous lactose, colloidal
silicon dioxide, corn starch, hypromellose, magnesium
stearate, microcrystalline cellulose, povidone and sodium
lauryl sulfate.
Manufactured by:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
S-10315 R1
Packaged and Distributed by:
UDL LABORATORIES, INC.
ROCKFORD, IL 61103
This unit dose package is not child resistant.
For institutional use only.
Keep this and all drugs out of the reach of children.
This container provides light-resistance.
See window for lot number and expiration date.

Loratadineloratadine TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||