Loperamide Hydrochloride
LOPERAMIDE HYDROCHLORIDE ORAL SOLUTION
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each 5 mL)
- Purpose
- Use
- Warnings
- Directions
- Loperamide Hydrochloride Other information
- Inactive ingredients
- How Supplied
- PRINCIPAL DISPLAY PANEL - 2 mg/10 mL Cup Lid Label
- PRINCIPAL DISPLAY PANEL - 1 mg/5 mL Cup Lid Label
FULL PRESCRIBING INFORMATION
1 mg/5 mL
2 mg/10 mL
Cherry Mint Flavor
For Hospital Use Only
Drug Facts
Active ingredient (in each 5 mL)
Loperamide HCl 1 mg
Purpose
Anti-diarrheal
Use
controls symptoms of diarrhea, including Travelers' Diarrhea
Warnings
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to Loperamide HCl
Do not use if you have bloody or black stool
Ask a doctor before use if you have
- fever
- mucus in the stool
- a history of liver disease
Ask a doctor or pharmacist before use if you are taking antibiotics
When using this product
- tiredness, drowsiness or dizziness may occur. Be careful when driving or operating machinery.
Stop use and ask a doctor if
- symptoms get worse
- diarrhea last for more than 2 days
- you get abdominal swelling or bulging. These may be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
use as directed per healthcare professional
Loperamide Hydrochloride Other information
- contains 0.5% alcohol
- store between 20° - 25°C (68° - 77°F)
- see individual label or shipper label for lot number and expiration date
Inactive ingredients
alcohol (0.5%), benzoic acid, citric acid, flavor, glycerin, propylene glycol, purified water, sodium benzoate, sorbitol, sucrose
How Supplied
NDC 68094-106-62
5 mL per unit dose cup
Thirty (30) cups per shipper
NDC 68094-217-62
10 mL per unit dose cup
Thirty (30) cups per shipper
Distributed By:
Perrigo Company
515 Eastern Avenue
Allegan, MI 49010
Packaged By:
Precision Dose, Inc.
722 Progressive Lane
S. Beloit, IL 61080
LI753
Rev. 05/09
PRINCIPAL DISPLAY PANEL - 2 mg/10 mL Cup Lid Label
NDC 68094-217-59
PrecisionDose™
LOPERAMIDE Hydrochloride
Oral Solution
2 mg/10 mL
Delivers 10 mL Contains 0.5% Alcohol
Each 5 mL contains 1 mg Loperamide HCl
Store at 20°-25° (68°-77°F)
Pkg. By: Precision Dose, Inc.
S. Beloit, IL 61080

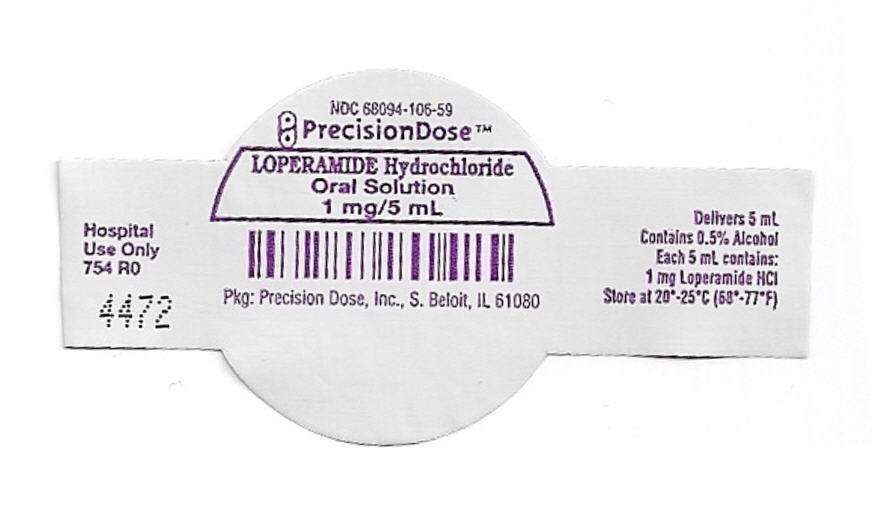
PRINCIPAL DISPLAY PANEL - 1 mg/5 mL Cup Lid Label
NDC 68094-106-59
PrecisionDose™
LOPERAMIDE Hydrochloride
Oral Solution
1 mg/5 mL
Pkg: Precision Dose, Inc., S. Beloit, IL 6180
Loperamide HydrochlorideLoperamide Hydrochloride SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Loperamide HydrochlorideLoperamide Hydrochloride SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||