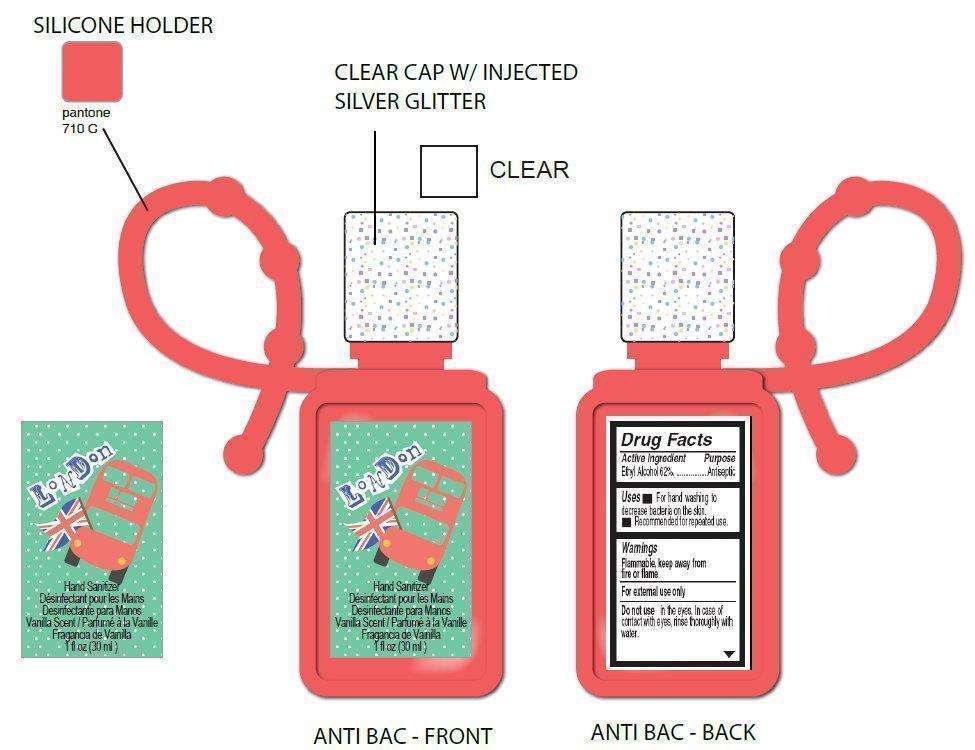
LONDON Hand Sanitizer Vanilla Scent
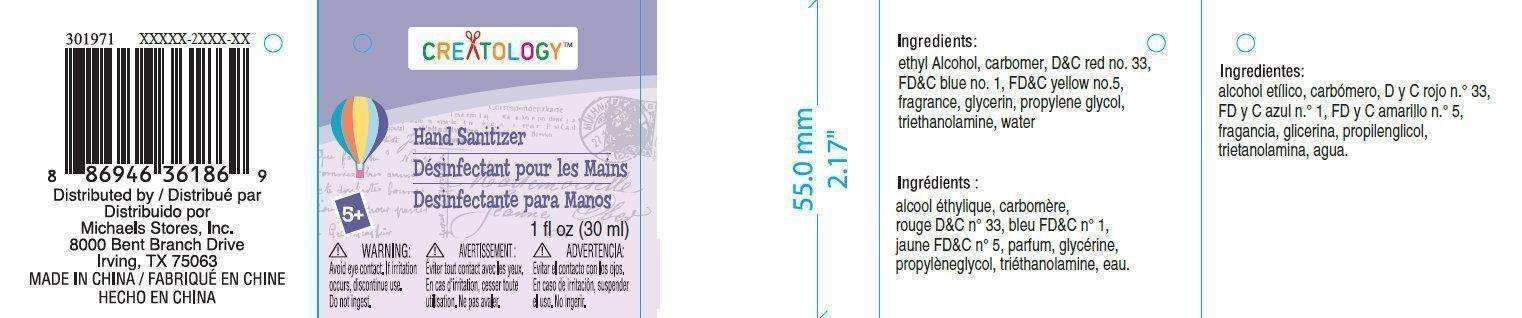
Michaels Stores Procurement Company
LONDON Hand Sanitizer Vanilla Scent
FULL PRESCRIBING INFORMATION: CONTENTS*
- LONDON Hand Sanitizer Vanilla Scent
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
- LONDON Hand Sanitizer Vanilla Scent Other information
- Inactive Ingredients
- LONDON Hand Sanitizer Vanilla Scent
FULL PRESCRIBING INFORMATION
LONDON Hand Sanitizer Vanilla Scent
Active Ingredient
Ethyl Alcohol 62%
Purpose
Antiseptic
Use
- For hand washing to decrease bacteria on the skin
- Recommended for repeated use.
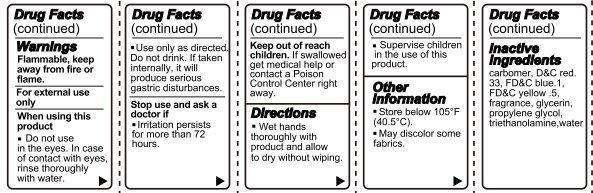
Warnings
- Flammable, keep away from fire or flame.
- For external use only.
- Do not use in the eyes. In case of contact with eyes, rinse thoroughly with water.
- Use only as directed. Do not drink. If taken internally, it will produce serious gastric disturbances.
- Stop use and ask a doctor if irritation persists for more than 72 hours.
-
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Wet hands thoroughly with product and allow to dry without wiping.
- Supervise children in the use of this product.
LONDON Hand Sanitizer Vanilla Scent Other information
- Store below 105 degrees F (40.5 degrees C).
- May discolor some fabrics.
Inactive Ingredients
- carbomer, DandC red n 33, FDandC blue n 1, FDandC yellow n 5, fragrance, glycerin, propylene glycol, triethanolamine, water
LONDON Hand Sanitizer Vanilla Scent

LONDON Hand Sanitizer Vanilla ScentALCOHOL GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!