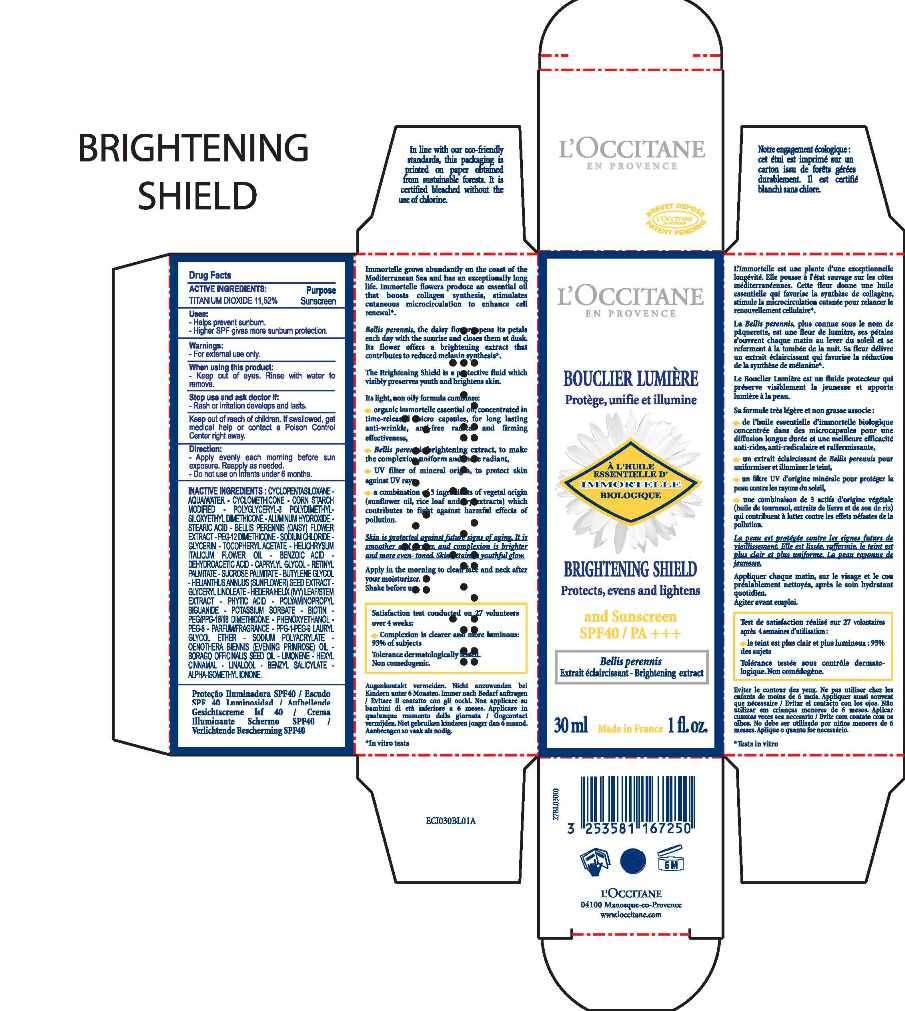
LOCCITANE
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active ingredient
ACTIVE INGREDIENTS PURPOSE
TITANIUM DIOXIDE 11,52% Sunscreen
LOCCITANE Uses
• helps prevent sunburn
• higher SPF gives more sunburn protection
Warnings
For external use only
When using this product
• keep out of eyes. Rinse with water to remove
Stop use and ask a doctor if
• rash or irritation develops and lasts
Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Directions
• apply evenly each morning before sun exposure
• do not use on infants under 6 months
• reapply as needed
Inactive ingredients
CYCLOPENTASILOXANE - AQUA/WATER - CYCLOMETHICONE - CORN STARCH MODIFIED - POLYGLYCERYL-3 POLYDIMETHYLSILOXYETHYL DIMETHICONE - ALUMINUM HYDROXIDE - STEARIC ACID - BELLIS PERENNIS (DAISY) FLOWER EXTRACT - PEG-12 DIMETHICONE - 'SODIUM CHLORIDE - GLYCERIN - TOCOPHERYL ACETATE - HELICHRYSUM ITALICUM FLOWER EXTRACT - BENZOIC ACID - DEHYDROACETIC ACID - CAPRYLYL GLYCOL - RETINYL PALMITATE - SUCROSE PALMITATE - BUTYLENE GLYCOL - HELIANTHUS ANNUUS (SUNFLOWER) SEED EXTRACT - GLYCERYL LINOLEATE - HEDERA HELIX (IVY) LEAF/STEM EXTRACT - PHYTIC ACID - POLYAMINOPROPYL BIGUANIDE - POTASSIUM SORBATE - BIOTIN - PEG/PPG-18/18 DIMETHICONE - PHENOXYETHANOL - PEG-8 - PARFUM/FRAGRANCE - PPG-1-PEG-9 LAURYL GLYCOL ETHER - SODIUM POLYACRYLATE - OENOTHERA BIENNIS (EVENING PRIMROSE) OIL - BORAGO OFFICINALIS SEED OIL - LIMONENE** - HEXYL CINNAMAL - LINALOOL - BENZYL SALICYLATE - ALPHA-ISOMETHYL IONONE
BOX
LOCCITANETITANIUM DIOXIDE CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||