Lithium Carbonate
Hetero Drugs Limited
Hetero Labs Limited
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING
- LITHIUM CARBONATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- LITHIUM CARBONATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LITHIUM CARBONATE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
WARNING
DOSAGE AND ADMINISTRATION
LITHIUM CARBONATE DESCRIPTION
Inactive Ingredients
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
LITHIUM CARBONATE CONTRAINDICATIONS
WARNINGS
DOSAGE AND ADMINISTRATION
Unmasking of Brugada Syndrome
Pregnancy
Lithium Induced Renal Effects
PRECAUTIONS
General
DOSAGE AND ADMINISTRATION
Information for Patients
Drug Interactions
Combined Use Of Haloperidol and Lithium.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDS):
Pregnancy, Teratogenic Effects
WARNINGS
Nursing Mothers
Usage in Children
Since information regarding the safety and effectiveness of lithium in children under 12 years of age is not available, its use in such patients is not recommended at this time. There has been a report of a transient syndrome of acute dystonia and hyperreflexia occurring in a 15 kg child who ingested 300 mg of lithium carbonate.
LITHIUM CARBONATE ADVERSE REACTIONS
Lithium Toxicity
Neuromuscular:
Central Nervous System:
Cardiovascular:
WARNINGS: Unmasking of Brugada Syndromeand PRECAUTIONS: Information for the Patients
Neurological:
Gastrointestinal:
Genitourinary:
Dermatologic:
Autonomic Nervous System:
Thyroid Abnormalities:
PRECAUTIONS
EEG Changes:
EKG Changes:
Miscellaneous:
Miscellaneous Reactions Unrelated to Dosage are:
OVERDOSAGE
ADVERSE REACTIONS
Treatment
DOSAGE & ADMINISTRATION
Acute Mania
Long-Term Control
N.B.
HOW SUPPLIED
Lithium Carbonate Capsules USP
150 mg White/White hard gelatin capsules (size 4)
300 mg Pink/Pink hard gelatin capsules (size 1)
600 mg Pink/White hard gelatin capsules (size ‘0EL’)
Store and Dispense:

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
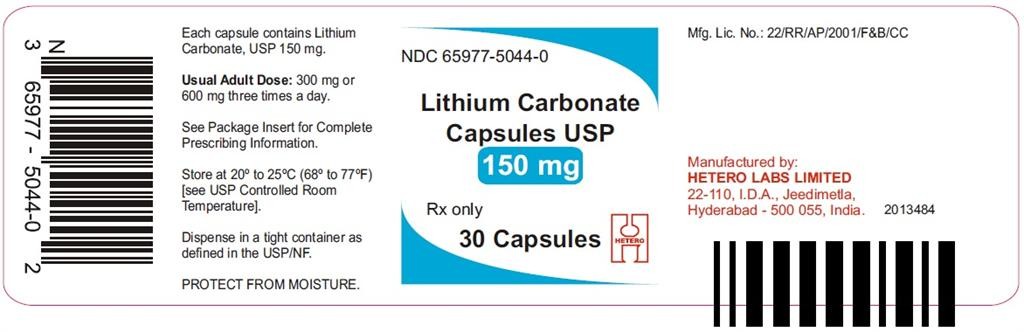




Lithium CarbonateLithium Carbonate CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Lithium CarbonateLithium Carbonate CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Lithium CarbonateLithium Carbonate CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!