Listerine Whitening Fluoride Anticavity
Johnson & Johnson Healthcare Products, Division of McNEIL-PPC, Inc.
Listerine Whitening Fluoride Anticavity Toothpaste Original Gel
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Listerine Whitening Fluoride Anticavity Other information
- Inactive ingredients
- Questions?
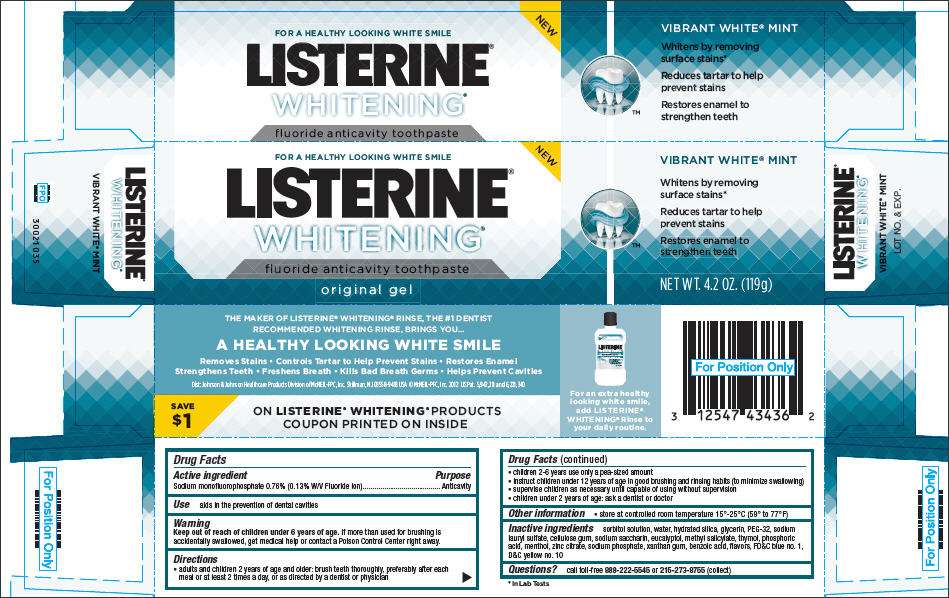
- PRINCIPAL DISPLAY PANEL - 119g Tube Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Sodium Monofluorophosphate 0.76% (0.13% w/v fluoride ion)
Purpose
Anticavity
Use
aids in the prevention of dental cavities
Warnings
Keep out of reach of children under 6 years of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 years of age and older: brush teeth thoroughly, preferably after each meal or at least 2 times a day, or as directed by a dentist or physician
- children 2-6 years use only a pea size amount
- Instruct children under 12 years of age in good brushing and rinsing habits (to minimize swallowing)
- supervise children as necessary until capable of using without supervision
- children under 2 years of age: ask a dentist or doctor
Listerine Whitening Fluoride Anticavity Other information
- store at (59° - 77°F)
Inactive ingredients
sorbitol solution, water, hydrated silica, glycerin, PEG-32, sodium lauryl sulfate, cellulose gum, sodium saccharin, eucalyptol, methyl salicylate, thymol, phosphoric acid, menthol, zinc citrate, sodium phosphate, xanthan gum, benzoic acid, flavors, FD&C blue no. 1, D&C yellow no. 10
Questions?
Call toll-free 888-222-0182 or 215-273-8755 (collect)
PRINCIPAL DISPLAY PANEL - 119g Tube Carton
NEW
FOR A HEALTHY LOOKING WHITE SMILE
LISTERINE®
WHITENING®
fluoride anticavity toothpaste
original gel
VIBRANT WHITE® MINT
Whitens by removing
surface stains®
NET WT. 4.2 OZ. (119g)
Listerine Whitening Fluoride AnticavitySodium Monofluorophosphate GEL, DENTIFRICE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||