Lisinopril
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING
- LISINOPRIL DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- LISINOPRIL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LISINOPRIL ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
WARNING
FETAL TOXICITYSee full prescribing information for complete boxed warning.
When pregnancy is detected, discontinue lisinopril tablets as soon as possible.
Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See WARNINGS, Fetal Toxicity.
LISINOPRIL DESCRIPTION
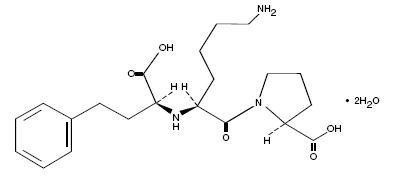
CLINICAL PHARMACOLOGY
Mechanism of ActionLisinopril inhibits angiotensin-converting enzyme (ACE) in human subjects and animals. ACE is a peptidly dispeptidase that catalyzes the conversion of angiotension I to the vasoconstrictor substance, angiotensin II. Angiotension II also stimulates aldosterone secretion by the adrenal cortex. The beneficial effects of lisinopril in hypertension and heart failure appear to result primarily from suppression of the renin-angiotensin-aldosterone system.Inhibition of ACE results in decreased plasma angiotensin II which leads to decreased vasopressor activity and to decreased aldosterone secretion. The latter decrease may result in a small increase of serum potassum. In hypertensive patiens with normal renal function treated with lisinopril alone for up to 24 weeks, the mean increase in serum potassium was approximately 0.1 mEq/L; however, approximately 15% of patients had increases greater than 0.5 mEq/L and approximately 6% had a decrease greater than 0.5 mEq/L and approximately 12% had a decrease greater than 0.5 mEq/L (seePRECAUTIONS). Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity.
Pharmacokinetics and Metabolism
Adult Patients
DOSAGE AND ADMINISTRATION). Lisinopril can be removed by hemodialysis.
Pediatric Patients
Pharmacodynamics and Clinical Effects
Hypertension
WARNINGS). When given together with thiazide-type diuretics, the blood pressure lowering effects of the two drugs are approximately additive.
PRECAUTIONS).
DOSAGE AND ADMINISTRATION, Preparation of Suspension (for 200 mL of a 1 mg/mL Suspension)).
Heart Failure
Acute Myocardial Infarction
DOSAGE AND ADMINISTRATION).
ADVERSE REACTIONS, Acute Myocardial Infarction).
INDICATIONS & USAGE
HypertensionHeart Failure
Acute Myocardial Infarction
WARNINGS).
WARNINGS, Anaphylactoid and Possibly Related Reactions).
LISINOPRIL CONTRAINDICATIONS
WARNINGS
Anaphylactoid and Possibly Related ReactionsHead and Neck Angioedema
Where there is involvement of the tongue, glottis or larynx, likely to cause airway obstruction, appropriate therapy, e.g., subcutaneous epinephrine solution 1:1000 (0.3 mL to 0.5 mL) and/or measures necessary to ensure a patent airway should be promptly provided (seeADVERSE REACTIONS).
Intestinal Angioedema
Intestinal angiodema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vimiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angiedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain.
INDICATIONS AND USAGE and CONTRAINDICATIONS).
Anaphylactoid Reactions During Desensitization
Anaphylactoid Reactions During Membrane Exposure
Hypotension
DOSAGE AND ADMINISTRATION).
PRECAUTIONS, Drug Interactions, andADVERSE REACTIONS).
Leukopenia/Neutropenia/Agranulocytosis
Hepatic Failure
Fetal Toxicity
Pregnancy Category D
PRECAUTIONS,Pediatric Use).
PRECAUTIONS
GeneralAortic Stenosis/Hypertrophic Cardiomyopathy
Impaired Renal Function
Evaluation of patients with hypertension, heart failure, or myocardial infarction should always include assessment of renal function (seeDOSAGE AND ADMINISTRATION).
Hyperkalemia
Cough
Surgery/Anesthesia
Information for Patients
Angioedema
Symptomatic Hypotension
Hyperkalemia
Hypoglycemia
PRECAUTIONS, Drug Interactions).
Leukopenia/Neutropenia
Pregnancy
NOTE:As with many other drugs, certain advice to patients being treated with lisinopril is warranted. This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
Drug Interactions
Hypotension-Patients on Diuretic Therapy
WARNINGSandDOSAGE AND ADMINISTRATION). When a diuretic is added to the therapy of a patient receiving lisinopril, an additional antihypertensive effect is usually observed. Studies with ACE inhibitors in combination with diuretics indicate that the dose of the ACE inhibitor can be reduced when it is given with a diuretic (seeDOSAGE AND ADMINISTRATION).
Antidiabetics
Non-Steroidal Anti-Inflammatory Agents Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
Other Agents
Agents Increasing Serum Potassium
Lithium
Gold
Carcinogenesis, Mutagenesis, Impairment of Fertility
Nursing Mothers
Pediatric Use
Neonates with a history of in utero exposure to lisinopril:
CLINICAL PHARMACOLOGY, Pharmacokineticsand MetabolismandPharmacodynamics and Clinical Effects,andDOSAGE AND ADMINISTRATION).
Geriatric Use
CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, Heart Failure and CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, Acute Myocardial Infarction).
CLINICAL PHARMACOLOGY, Pharmacokinetics and Metabolism).
DOSAGE AND ADMINISTRATION).
LISINOPRIL ADVERSE REACTIONS
Hypertension
PERCENT OF PATIENTS IN CONTROLLED STUDIES
Lisinopril
(n = 1349)
Incidence (discontinuation)Lisinopril/Hydrochlorothiazide (n = 629)
Incidence (discontinuation) Placebo
(n = 207)
Incidence (discontinuation) Body As A Whole Cardiovascular Digestive Musculoskeletal Nervous/Psychiatric Respiratory Skin Urogenital
Heart Failure
CONTROLLED TRIALS
Lisinopril
(n = 407)
Incidence
(discontinuation)
12 weeks Placebo
(n = 155)
Incidence(discontinuation)
12 weeks Body As A Whole Cardiovascular Digestive Nervous/Psychiatric Respiratory Skin
*
% of Patients
Events High Dose (n = 1568) Low Dose (n = 1596)
*increased
Acute Myocardial Infarction
Body as a Whole
WARNINGS,Anaphylactoid and Possibly Related Reactions), syncope, orthostatic effects, chest discomfort, pain, pelvic pain, flank pain, edema, facial edema, virus infection, fever, chills, malaise
Cardiovascular
WARNINGS,Hypotension); pulmonary embolsim and infarction, arrhythmias (including ventricular tachycardia, atrial tachycardia, atrial fibillation, bradycardia and premature ventricular contractions), palpitations, transient ischemic attacks, paroxysmal nocturnal dyspnea, orthostatic hypotension, decreased blood pressure, peripheral edema, vasculitis
Digestive
WARNINGS,Hepatic Failure), vomiting, gastritis, dyspepsia, heartburn, gastrointestinal cramps, constipation, flatulence, dry mouth
Hematologic
Endocrine
Metabolic
PRECAUTIONS, Drug Interactions).
Musculoskeletal
Nervous System/Psychiatric
Respiratory System
Skin
Special Senses
Urogenital System
PRECAUTIONSandDOSAGE AND ADMINISTRATION), pyelonephritis, dysuria, urinary tract infection, breast pain
Miscellaneous
Angioedema
Hypotension
WARNINGS).
Cough
PRECAUTIONS,Cough.
Pediatric Patients
Clinical Laboratory Test Findings
Serum Electrolytes
PRECAUTIONS), hyponatremia
Creatinine, Blood Urea Nitrogen
PRECAUTIONS). Reversible minor increases in blood urea nitrogen and serum creatinine were observed in approximately 11.6% of patients with heart failure on concomitant diuretic therapy. Frequently, these abnormalities resolved when the dosage of the diuretic was decreased.
Hemoglobin and Hematocrit
Liver Function Tests
WARNINGS, Hepatic Failure).
OVERDOSAGE
WARNINGS,AnaphylactoidReactions During Membrane Exposure).
DOSAGE & ADMINISTRATION
HypertensionInitial Therapy
Diuretic Treated Patients
WARNINGS). The dosage of lisinopril tablets USP should be adjusted according to blood pressure response. If the patientblood pressure is not controlled with lisinopril tablets USP alone, diuretic therapy may be resumed as described above.
WARNINGSandPRECAUTIONS, Drug Interactions).
Dosage Adjustment in Renal Impairment
*
WARNINGS, Anaphylactoid ReactionsDuring Membrane Exposure.
*
Heart Failure
WARNINGSandPRECAUTIONS, Drug Interactions). The appearance of hypotension after the initial dose of lisinopril tablets USP does not preclude subsequent careful dose titration with the drug, following effective management of the hypotension.
Dosage Adjustment in Patients With Heart Failure and Renal Impairment or Hyponatremia
WARNINGSandPRECAUTIONS, Drug Interactions).
Acute Myocardial Infarction
WARNINGS). If hypotension occurs (systolic blood pressure100 mmHg), a daily maintenance dose of 5 mg may be given with temporary reductions to 2.5 mg if needed. If prolonged hypotension occurs (systolic blood pressure < 90 mmHg for more than 1 hour) lisinopril tablets USP should be withdrawn. For patients who develop symptoms of heart failure, seeDOSAGE AND ADMINISTRATION, Heart Failure.
Dosage Adjustment in Patients With Myocardial Infarction With Renal Impairment
Use in Elderly
Pediatric Hypertensive Patients6 Years of Age
CLINICAL PHARMACOLOGY,PharmacokineticsandMetabolism and Pharmacodynamics and Clinical Effects).
CLINICAL PHARMACOLOGY, Pharmacokinetics and Metabolism and Pharmacodynamics and Clinical Effects andPRECAUTIONS).
Preparation of Suspension (for 200 mL of a 1 mg/mL Suspension)
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

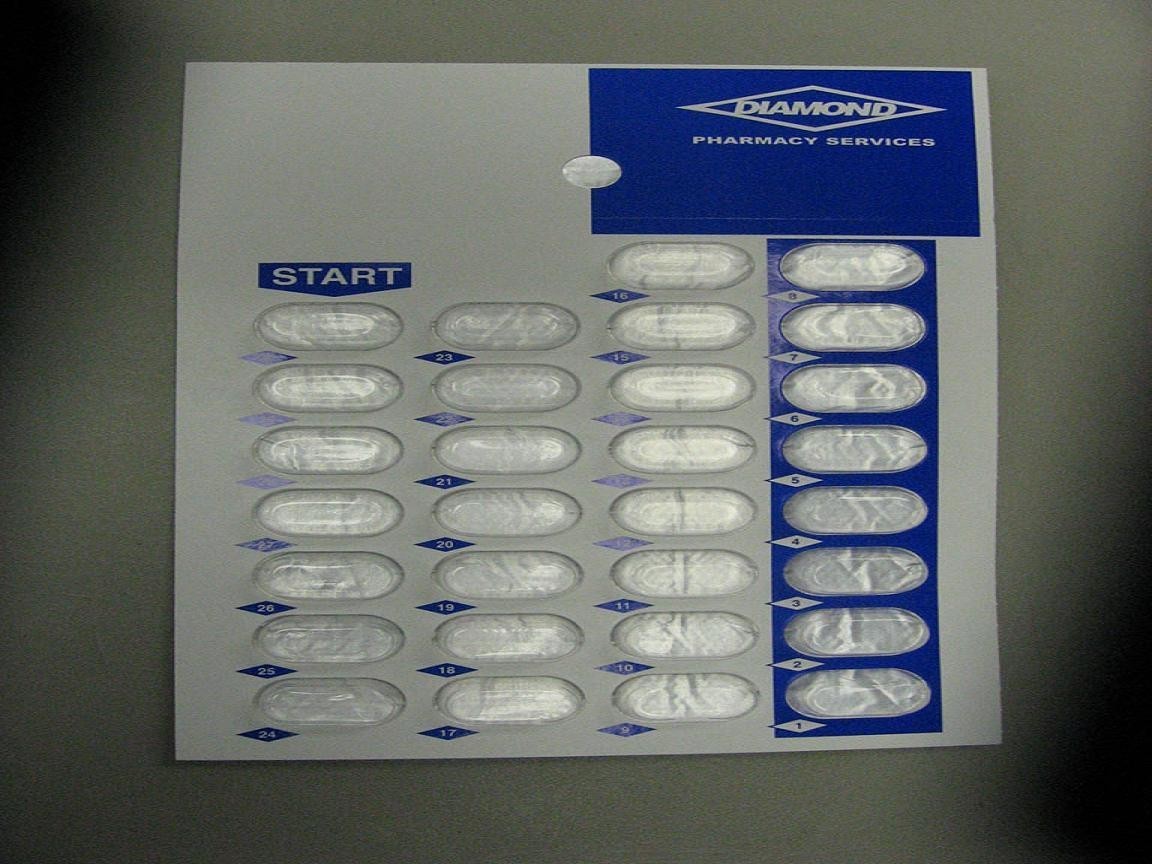
LisinoprilLISINOPRIL TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!