Lisinopril and Hydrochlorothiazide
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- LISINOPRIL AND HYDROCHLOROTHIAZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- PHARMACODYNAMICS
- INDICATIONS & USAGE
- LISINOPRIL AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- LISINOPRIL AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
LISINOPRIL AND HYDROCHLOROTHIAZIDE DESCRIPTION
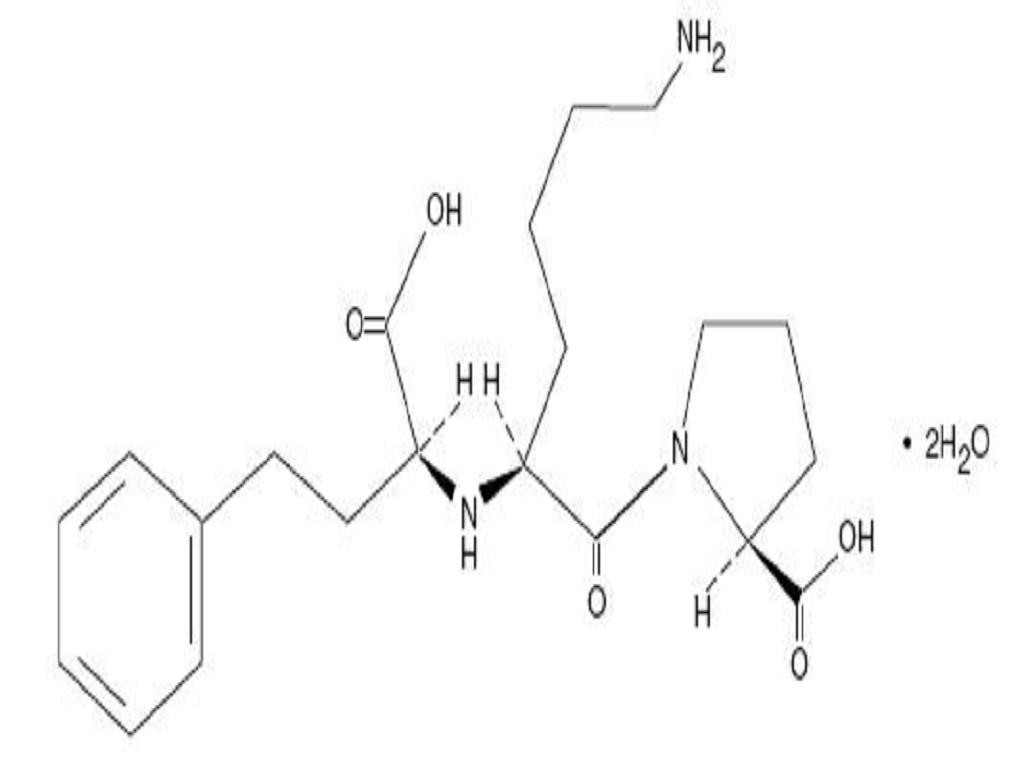
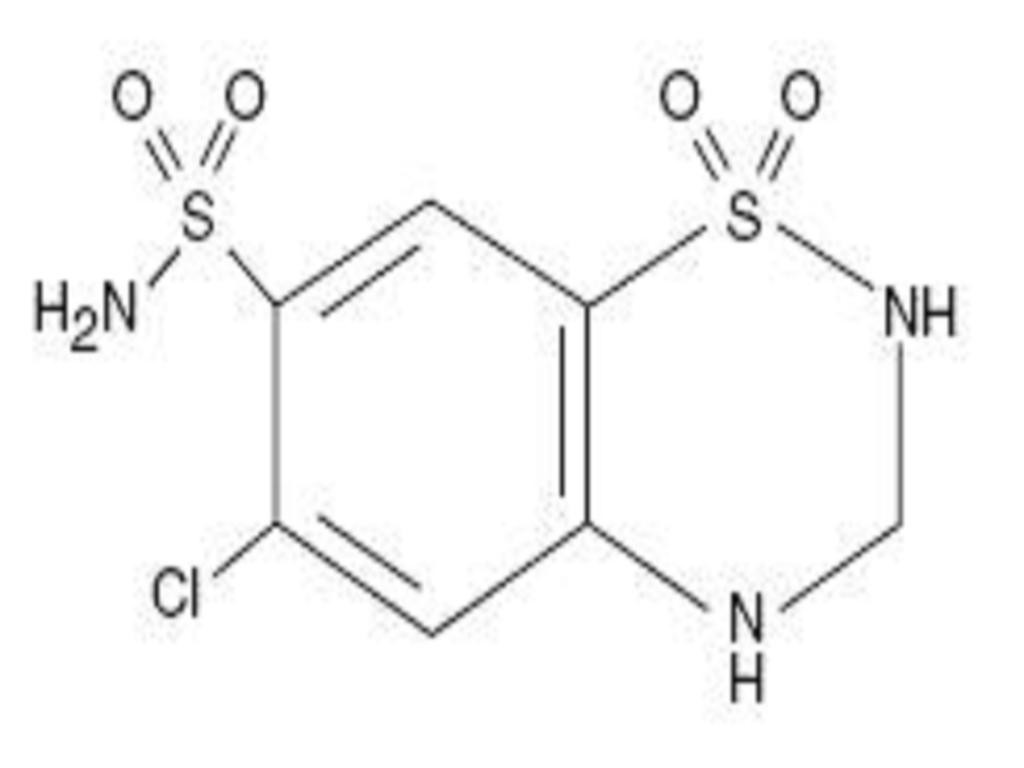
CLINICAL PHARMACOLOGY
Lisinopril and HydrochlorothiazideLisinopril
Mechanism of Action
PHARMACOKINETICS
Pharmacokinetics and MetabolismPHARMACODYNAMICS
Hydrochlorothiazide
INDICATIONS & USAGE
LISINOPRIL AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
WARNINGS
LisinoprilAnaphylactoid and Possibly Related Reactions
Head and Neck Angioedema
Intestinal Angioedema
Anaphylactoid Reactions During Desensitization
Anaphylactoid Reactions During Membrane Exposure
Hypotension and Related Effects
Leukopenia/Neutropenia/Agranulocytosis
Hepatic Failure
Pregnancy
Lisinopril and Hydrochlorothiazide
Lisinopril
Fetal/Neonatal Morbidity and Mortality
Hydrochlorothiazide
Acute Myopia and Secondary Angle-Closure Glaucoma
Teratogenic Effects
Nonteratogenic Effects
Hydrochlorothiazide
PRECAUTIONS
GeneralLisinopril
Aortic Stenosis/Hypertrophic Cardiomyopathy
Impaired Renal Function
Hyperkalemia
Cough
Surgery/Anesthesia
Hydrochlorothiazide
INFORMATION FOR PATIENTS
AngioedemaSymptomatic Hypotension
Hyperkalemia
Leukopenia/Neutropenia
Pregnancy
DRUG INTERACTIONS
LisinoprilHypotension - Patients on Diuretic Therapy
Non-steroidal Anti-inflammatory Agents
Other Agents
Agents Increasing Serum Potassium
Lithium
Hydrochlorothiazide
Alcohol, Barbiturates, or Narcotics
Antidiabetic Drugs (oral agents and insulin)
Other Antihypertensive Drugs
Cholestyramine and Colestipol Resins
Corticosteroids, ACTH
Pressor Amines (e.g., norepinephrine)
Skeletal Muscle Relaxants, Nondepolarizing (e.g., tubocurarine)
Lithium
Non-steroidal Anti-inflammatory Drugs
Gold
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Lisinopril and HydrochlorothiazideLisinopril
Hydrochlorothiazide
PREGNANCY
Pregnancy Categories C (first trimester) and D (second and third trimesters)NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
LISINOPRIL AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
Angioedema
Hypotension
Cough
Clinical Laboratory Test Findings
Serum Electrolytes
Creatinine, Blood Urea Nitrogen
Serum Uric Acid, Glucose, Magnesium, Cholesterol, Triglycerides and Calcium
Hemoglobin and Hematocrit
Liver Function Tests
Lisinopril
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic
Metabolic
Musculoskeletal
Nervous System/Psychiatric
Respiratory
Skin
Special Senses
Urogenital
Miscellaneous
Fetal/Neonatal Morbidity and Mortality
Hydrochlorothiazide
Body as a Whole
Digestive
Hematologic
Musculoskeletal
Nervous System/Psychiatric
Renal
Skin
Special Senses
Hypersensitivity
DOSAGE & ADMINISTRATION
Dose Titration Guided by Clinical Effect
Replacement Therapy
Use in Renal Impairment
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Lisinopril and HydrochlorothiazideLisinopril and Hydrochlorothiazide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!