Lisinopril
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- LISINOPRIL DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS AND METABOLISM
- PHARMACODYNAMICS AND CLINICAL EFFECTS:
- INDICATIONS & USAGE
- LISINOPRIL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- LISINOPRIL ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
USE IN PREGNANCYWhen used in pregnancy during the second and third trimesters, ACE inhibitors can cause injury and even death to the developing fetus.WARNINGS, Fetal/Neonatal Morbidity and Mortality
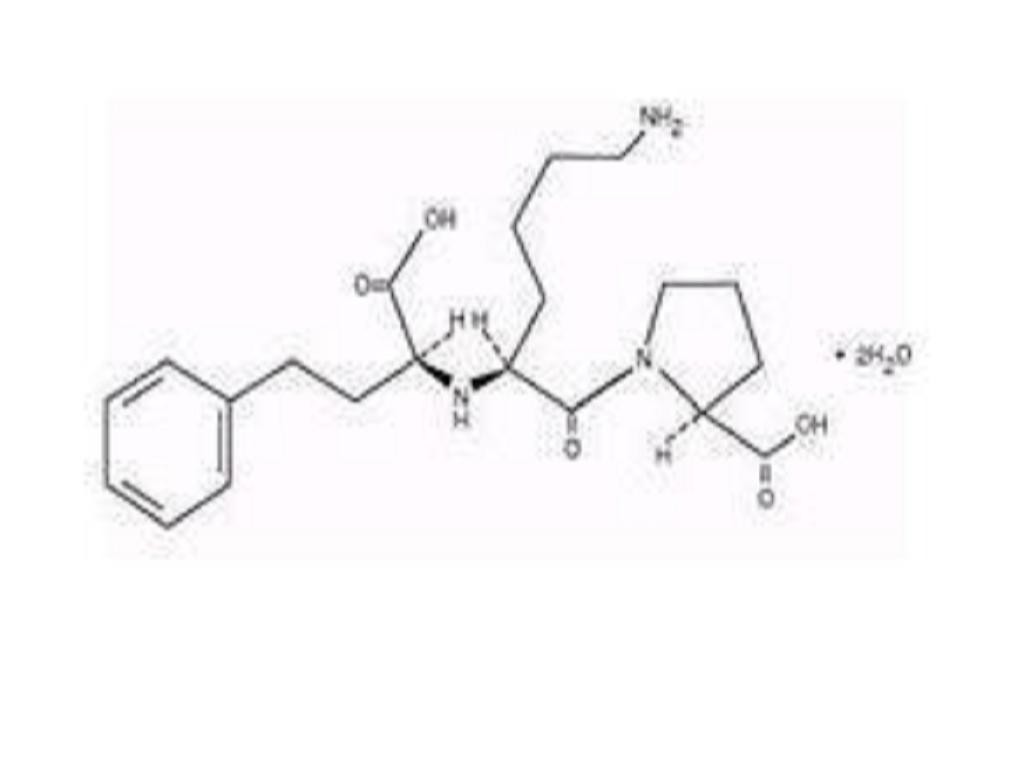
LISINOPRIL DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanism of Action:PRECAUTIONSPHARMACOKINETICS AND METABOLISM
DOSAGE AND ADMINISTRATION
PHARMACODYNAMICS AND CLINICAL EFFECTS:
Hypertension:WARNINGS
PRECAUTIONS
DOSAGE AND ADMINISTRATION
Heart Failure:
Acute Myocardial Infarction:
DOSAGE AND ADMINISTRATION
ADVERSE REACTIONS - Acute Myocardial Infarction
INDICATIONS & USAGE
Hypertension:Heart Failure
Acute Myocardial Infarction:
WARNINGS
WARNINGS, Anaphylactoid and Possibly Related Reactions
LISINOPRIL CONTRAINDICATIONS
WARNINGS
Anaphylactoid and Possibly Related Reactions:Head and Neck Angioedema:
Where there is involvement of the tongue, glottis or larynx, likely to cause airway obstruction, appropriate therapy, e.g., subcutaneous epinephrine solution 1:1000 (0.3 mL to 0.5 mL) and/or measures necessary to ensure a patent airway should be promptly provided.ADVERSE REACTIONS
Intestinal Angioedema:
INDICATIONS AND USAGECONTRAINDICATIONS
Anaphylactoid Reactions During Desensitization:
Anaphylactoid Reactions During Membrane Exposure:
Hypotension:
DOSAGE AND ADMINISTRATION
PRECAUTIONS, Drug InteractionsADVERSE REACTIONS
Leukopenia/Neutropenia/Agranulocytosis:
Hepatic Failure:
Fetal/Neonatal Morbidity and Mortality:
PRECAUTIONS
General
Aortic Stenosis/Hypertrophic Cardiomyopathy:
Impaired Renal Function:
DOSAGE AND ADMINISTRATION
Hyperkalemia:
Drug Interactions
Cough:
Surgery/Anesthesia:
INFORMATION FOR PATIENTS
PRECAUTIONS, Drug Interactions
NOTE:
DRUG INTERACTIONS
WARNINGSDOSAGE AND ADMINISTRATIONDOSAGE AND ADMINISTRATIONCARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Pregnancy Categories C (first trimester) and D (second and third trimesters).WARNINGS, Fetal/Neonatal Morbidity and MortalityNURSING MOTHERS
PEDIATRIC USE
CLINICAL PHARMACOLOGY, Pharmacokinetics and MetabolismPharmacodynamics and Clinical EffectsDOSAGE AND ADMINISTRATION
GERIATRIC USE
CLINICAL PHARMACOLOGYPharmacodynamics and Clinical EffectsHeart FailureCLINICAL PHARMACOLOGYPharmacodynamics and Clinical EffectsAcute Myocardial Infarction
CLINICAL PHARMACOLOGYPharmacokinetics and Metabolism
DOSAGE AND ADMINISTRATION
LISINOPRIL ADVERSE REACTIONS
Hypertension
Heart Failure
Acute Myocardial Infarction
WARNINGS, Anaphylactoid Reactions and Possibly Related Reactions
WARNINGS, Hypotension
Digestive: Pancreatitis, hepatitis (hepatocellular or cholestatic jaundice) (seeWARNINGS, Hepatic Failure), vomiting, gastritis, dyspepsia, heartburn, gastrointestinal cramps, constipation, flatulence, dry mouth.
Hematologic: Rare cases of bone marrow depression, hemolytic anemia, leukopenia/neutropenia and thrombocytopenia.
Endocrine: Diabetes mellitus.
Metabolic: Weight loss, dehydration, fluid overload, gout, weight gain.
Cases of hypoglycemia in diabetic patients on oral antidiabetic agents or insulin have been reported in post-marketing experience (SeePRECAUTIONS, Drug Interactions).
Musculoskeletal: Arthritis, arthralgia, neck pain, hip pain, low back pain, joint pain, leg pain, knee pain, shoulder pain, arm pain, lumbago.
Nervous System/Psychiatric: Stroke, ataxia, memory impairment, tremor, peripheral neuropathy (e.g., dysesthesia), spasm, paresthesia, confusion, insomnia, somnolence, hypersomnia, irritability and nervousness.
Respiratory System: Malignant lung neoplasms, hemoptysis, pulmonary infiltrates, bronchospasm, asthma, pleural effusion, pneumonia, eosinophilic pneumonitis, bronchitis, wheezing, orthopnea, painful respiration, epistaxis, laryngitis, sinusitis, pharyngeal pain, pharyngitis, rhinitis, rhinorrhea.
Skin: Urticaria, alopecia, herpes zoster, photosensitivity, skin lesions, skin infections, pemphigus, erythema, flushing, diaphoresis, cutaneous pseudolymphoma. Other severe skin reactions have been reported rarely, including toxic epidermal necrolysis and Stevens-Johnson syndrome; causal relationship has not been established.
Special Senses: Visual loss, diplopia, blurred vision, tinnitus, photophobia, taste disturbances.
Urogenital System: Acute renal failure, oliguria, anuria, uremia, progressive azotemia, renal dysfunction (seePRECAUTIONSandDOSAGE AND ADMINISTRATION), pyelonephritis, dysuria, urinary tract infection, breast pain.
Miscellaneous: A symptom complex has been reported which may include a positive ANA, an elevated erythrocyte sedimentation rate, arthralgia/arthritis, myalgia, fever, vasculitis, eosinophilia and leukocytosis. Rash, photosensitivity or other dermatological manifestations may occur alone or in combination with these symptoms.
Angioedema: Angioedema has been reported in patients receiving lisinopril (0.1%) with an incidence higher in Black than in non-Black patients. Angioedema associated with laryngeal edema may be fatal. If angioedema of the face, extremities, lips, tongue, glottis and/or larynx occurs, treatment with lisinopril should be discontinued and appropriate therapy instituted immediately. (SeeWARNINGS.)
In rare cases, intestinal angioedema has been reported in post marketing experience.
Hypotension: In hypertensive patients, hypotension occurred in 1.2% and syncope occurred in 0.1% of patients with an incidence higher in Black than in non-Black patients. Hypotension or syncope was a cause of discontinuation of therapy in 0.5% of hypertensive patients. In patients with heart failure, hypotension occurred in 5.3% and syncope occurred in 1.8% of patients. These adverse experiences were possibly dose-related (see above data from ATLAS Trial) and caused discontinuation of therapy in 1.8% of these patients in the symptomatic trials. In patients treated with lisinopril for six weeks after acute myocardial infarction, hypotension (systolic blood pressuremmHg) resulted in discontinuation of therapy in 9.7% of the patients. (SeeWARNINGS.)
Fetal/Neonatal Morbidity and Mortality: SeeWARNINGS, Fetal/Neonatal Morbidity and Mortality.
Cough: SeePRECAUTIONS Cough
Pediatric Patients: No relevant differences between the adverse experience profile for pediatric patients and that previously reported for adult patients were identified.
Clinical Laboratory Findings
Serum Electrolytes: Hyperkalemia (SeePRECAUTIONS), hyponatremia.
Creatinine, Blood Urea Nitrogen: Minor increases in blood urea nitrogen and serum creatinine, reversible upon discontinuation of therapy, were observed in about 2.0% of patients with essential hypertension treated with lisinopril alone. Increases were more common in patients receiving concomitant diuretics and in patients with renal artery stenosis. (SeePRECAUTIONS.) Reversible minor increases in blood urea nitrogen and serum creatinine were observed in approximately 11.6% of patients with heart failure on concomitant diuretic therapy. Frequently, these abnormalities resolved when the dosage of the diuretic was decreased.
Hemoglobin and Hematocrit: Small decreases in hemoglobin and hematocrit (mean decreases of approximately 0.4 g% and 1.3 vol%, respectively) occurred frequently in patients treated with lisinopril but were rarely of clinical importance in patients without some other cause of anemia. In clinical trials, less than 0.1% of patients discontinued therapy due to anemia. Hemolytic anemia has been reported; a causal relationship to lisinopril cannot be excluded.
Liver Function Tests: Rarely, elevations of liver enzymes and/or serum bilirubin have occurred. (SeeWARNINGS,Hepatic Failure.)
In hypertensive patients, 2.0% discontinued therapy due to laboratory adverse experiences, principally elevations in blood urea nitrogen (0.6%), serum creatinine (0.5%) and serum potassium (0.4%).
In the heart failure trials, 3.4% of patients discontinued therapy due to laboratory adverse experiences; 1.8% due to elevations in blood urea nitrogen and/or creatinine and 0.6% due to elevations in serum potassium.
In the myocardial infarction trial, 2.0% of patients receiving lisinopril discontinued therapy due to renal dysfunction (increasing creatinine concentration to over 3 mg/dL or a doubling or more of the baseline serum creatinine concentration); less than 1.0% of patients discontinued therapy due to other laboratory adverse experiences: 0.1% with hyperkalemia and less than 0.1% with hepatic enzyme alterations.
OVERDOSAGE
WARNINGSAnaphylactoid Reactions During Membrane Exposure
DOSAGE & ADMINISTRATION
Hypertension
WARNINGS
WARNINGSPRECAUTIONSDrug Interactions
PRECAUTIONS
WARNINGS, Anaphylactoid Reactions During Membrane Exposure
Heart Failure
WARNINGSPRECAUTIONS, Drug Interactions
WARNINGSPRECAUTIONS, Drug Interactions
Acute Myocardial Infarction
WARNINGSDOSAGE AND ADMINISTRATION, Heart Failure
Use in Elderly
Pediatric Hypertensive Patients6 years of age
CLINICAL PHARMACOLOGY, Pharmacokinetics and MetabolismPharmacodynamicsClinical Effects.
CLINICAL PHARMACOLOGY, Pharmacokinetics and MetabolismPharmacodynamics and Clinical EffectsPRECAUTIONS
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

LisinoprilLisinopril TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!