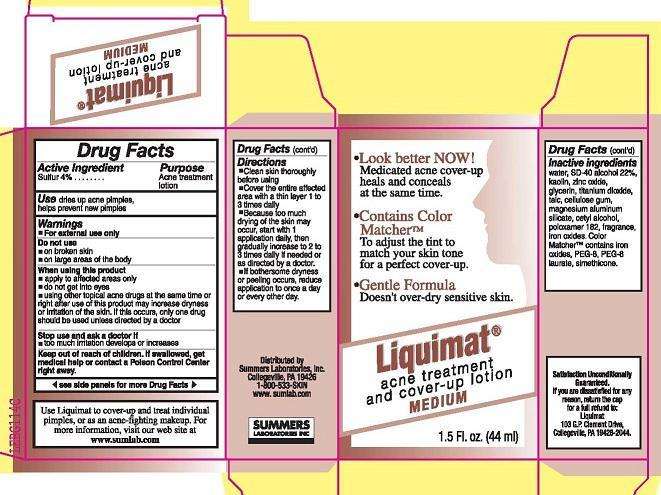
LIQUIMAT
FULL PRESCRIBING INFORMATION
Active ingredient
ACTIVE INGREDIENT
SULFUR 4%
Purpose
PURPOSE
ACNE TREATMENT LOTION
Uses
USE
DRIES UP ACNE PIMPLES, HELPS PREVENT NEW PIMPLES
WARNINGS
- FOR EXTERNAL USE ONLY
DO NOT USE
- ON BROKEN SKIN
- ON LARGE AREAS OF THE BODY
WHEN USING THIS PRODUCT
- APPLY TO AFFECTED AREAS ONLY
- DO NOT GET INTO EYES
- USING OTHER TOPICAL ACNE DRUGS AT THE SAME TIME OR RIGHT AFTER USE OF THIS PRODUCT MAY INCREASE DRYNESS OR IRRITATION OF THE SKIN. IF THIS OCCURS, ONLY ONE DRUG SHOULD BE USED UNLESS DIRECTED BY A DOCTOR
STOP USE AND ASK A DOCTOR IF
- TOO MUCH IRRITATION DEVELOPS OR INCREASES
KEEP OUT OF REACH OF CHILDREN. IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
DIRECTIONS
- CLEAN SKIN THOROUGHLY BEFORE USING
- COVER THE ENTIRE AFFECTED AREA WITH A THIN LAYER 1 TO 3 TIMES DAILY
- BECAUSE TOO MUCH DRYING OF THE SKIN MAY OCCUR, START WITH 1 APPLICATION DAILY, THEN INCREASE TO 2 TO 3 TIMES DAILY IF NEEDED OR AS DIRECTED BY A DOCTOR.
INACTIVE INGREDIENTS
WATER, SD-40 ALCOHOL 22-PERCENT, KAOLIN, ZINC OXIDE, GLYCERIN, TITANIUM DIOXIDE, TALC, CELLULOSE GUM, MAGNESIUM ALUMINUM SILICATE, CETYL ALCOHOL, POLOXAMER 182, FRAGRANCE, IRON OXIDES. COLOR MATCHER CONTAINS IRON OXIDES, PEG-8, PEG-8 LAURATE, SIMETHICONE.
LIQUIMATSULFUR LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!