Levocetirizine Dihydrochloride
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use levocetirizine dihydrochloride tablets safely and effectively. See full prescribing information for levocetirizine dihydrochloride tablets. Levocetirizine Dihydrochloride Tablets, 5 mgInitial U.S. Approval: 1995 RECENT MAJOR CHANGES5.2INDICATIONS AND USAGE1 The treatment of the uncomplicated skin manifestations of chronic idiopathic urticaria (1.3) DOSAGE AND ADMINISTRATION Adults and children 12 years of age and older: 5 mg once daily in the evening (2.1) Children 6 to 11 years of age: 2.5 mg once daily in the evening (2.2) Renal Impairment Adjust the dose in patients 12 years of age and older with decreased renal function (2.4, 12.3) DOSAGE FORMS AND STRENGTHS Immediate-release breakable (scored) tablets, 5 mg (3) CONTRAINDICATIONS Patients with a known hypersensitivity to levocetirizine or any of the ingredients of levocetirizine dihydrochloride tablet or to cetirizine (4) Patients with end-stage renal disease at less than 10 mL/min creatinine clearance or patients undergoing hemodialysis (4) Children 6 months to 11 years of age with renal impairment (4) WARNINGS AND PRECAUTIONS Avoid engaging in hazardous occupations requiring complete mental alertness such as driving or operating machinery when taking levocetirizine dihydrochloride tablets (5.1). Avoid concurrent use of alcohol or other central nervous system depressants with levocetirizine dihydrochloride tablets (5.1). Use with caution in patients with predisposing factors of urinary retention (e.g. spinal cord lesion, prostatic hyperplasia). Discontinue levocetirizine dihydrochloride tablets if urinary retention occurs (5.2). Side Effects6.1To report SUSPECTED ADVERSE REACTIONS, contact CARACO Pharmaceutical Laboratories Ltd. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.USE IN SPECIFIC POPULATIONS8.612.312.3Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.’s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 LEVOCETIRIZINE DIHYDROCHLORIDE INDICATIONS AND USAGE
- 2 LEVOCETIRIZINE DIHYDROCHLORIDE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 LEVOCETIRIZINE DIHYDROCHLORIDE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 LEVOCETIRIZINE DIHYDROCHLORIDE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 LEVOCETIRIZINE DIHYDROCHLORIDE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.3 Chronic Idiopathic Urticaria
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.’s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
2 DOSAGE AND ADMINISTRATION
2.1 Adults and Children 12 Years of Age and Older
2.2 Children 6 to 11 Years of Age
[see Clinical Pharmacology (12.3)].
2.3 Children 6 months to 5 Years of Age
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.’s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
2.4 Dose Adjustment for Renal and Hepatic Impairment
- Mild renal impairment (creatinine clearance [CLCR] = 50 to 80 mL/min): a dose of 2.5 mg once daily is recommended;
- Moderate renal impairment (CLCR = 30 to 50 mL/min): a dose of 2.5 mg once every other day is recommended;
- Severe renal impairment (CLCR = 10 to 30 mL/min): a dose of 2.5 mg twice weekly (administered once every 3 to 4 days) is recommended;
- End-stage renal disease patients (CLCR < 10 mL/min) and patients undergoing hemodialysis should not receive levocetirizine dihydrochloride tablets.
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Patients with known hypersensitivity
[see Adverse Reactions (6.2)].
4.2 Patients with end-stage renal disease
CR
4.3 Pediatric patients with impaired renal function
5 WARNINGS AND PRECAUTIONS
5.1 Somnolence
5.2 Urinary Retention
Urinary
6 ADVERSE REACTIONS
[see Warnings and Precautions (5)].
6.1 Clinical Trials Experience
Adults and Adolescents 12 years of Age and Older
| Adverse Reactions | Levocetirizine 2.5 mg (n = 421) |
Levocetirizine 5 mg (n = 1070) |
Placebo (n = 912) |
|---|---|---|---|
| Somnolence |
22 (5%) |
61 (6%) |
16 (2%) |
| Nasopharyngitis |
25 (6%) |
40 (4%) |
28 (3%) |
| Fatigue |
5 (1%) |
46 (4%) |
20 (2%) |
| Dry Mouth |
12 (3%) |
26 (2%) |
11 (1%) |
| Pharyngitis |
10 (2%) |
12 (1%) |
9 (1%) |
Pediatric Patients 6 to 12 Years of Age
| Adverse Reactions | Levocetirizine 5 mg (n = 243) |
Placebo (n = 240) |
|---|---|---|
| Pyrexia |
10 (4%) |
5 (2%) |
| Cough |
8 (3%) |
2 (<1%) |
| Somnolence |
7 (3%) |
1 (<1%) |
| Epistaxis |
6 (2%) |
1 (<1%) |
Long-Term Clinical Trials Experience
Laboratory Test Abnormalities
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
In vitro in vivo
7.1 Antipyrine, Azithromycin, Cimetidine, Erythromycin, Ketoconazole, Theophylline, and Pseudoephedrine
7.2 Ritonavir
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Teratogenic Effects:
2
8.3 Nursing Mothers
2
8.4 Pediatric Use
[see Clinical Studies (14)].
[see Adverse Reactions (6.1)].
[see Dosage and Administration (2.2); Clinical Studies (14); and Clinical Pharmacology (12.3)].
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.’s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
8.5 Geriatric Use
8.6 Renal Impairment
Dosage and Administration (2) and Clinical Pharmacology (12.3)
8.7 Hepatic Impairment
[see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
22
11 DESCRIPTION
1212523
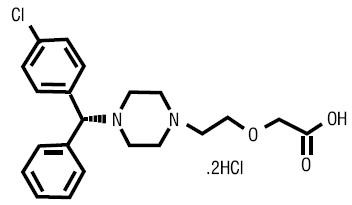
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
1In vitro1vs.
12.2 Pharmacodynamics
12.3 Pharmacokinetics
• Absorption
maxmax
• Distribution
in vitro
• Metabolism
• Elimination
Dosage and Administration2.3
• Drug Interaction Studies
In vitromax
in vivoDrug Interactions (7)
• Pediatric Patients
maxmax
Dedicated pharmacokinetic studies have not been conducted in pediatric patients younger than 6 years of age.
Pharmacokinetic information in pediatric patients (age 1 to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.’s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
• Geriatric Patients
Dosage and Administration (2)
• Gender
• Race
• Renal Impairment
CR
Dosage and Administration (2.4)
• Hepatic Impairment
Dosage and Administration (2)
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
2 22
in vivo
2
13.2 Animal Toxicology
2
2
14 CLINICAL STUDIES
14.2 Chronic Idiopathic Urticaria
Adult Patients 18 Years of Age and Older
| Treatment | N | Baseline | On Treatment Adjusted Mean | Difference from Placebo | ||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | p-value | ||||
|
Dose-Ranging Trial – Reflective pruritus severity score
|
||||||
| Levocetirizine 2.5 mg |
69 |
2.08 |
1.02 |
0.82 |
(0.58, 1.06) |
<0.001 |
| Levocetirizine 5 mg |
62 |
2.07 |
0.92 |
0.91 |
(0.66, 1.16) |
<0.001 |
| Levocetirizine 10 mg |
55 |
2.04 |
0.73 |
1.11 |
(0.85, 1.37) |
<0.001 |
| Placebo |
60 |
2.25 |
1.84 |
|||
|
Chronic Idiopathic Urticaria Trial – Reflective pruritus severity score
|
||||||
| Levocetirizine 5 mg |
80 |
2.07 |
0.94 |
0.62 |
(0.38, 0.86) |
<0.001 |
| Placebo |
82 |
2.06 |
1.56 |
|||
[see Use in Specific Populations (8.4)].
16 HOW SUPPLIED/STORAGE AND HANDLING
Storage:
17 PATIENT COUNSELING INFORMATION
17.1 Somnolence
17.2 Concomitant Use of Alcohol and other Central Nervous System Depressants
17.3 Dosing of Levocetirizine Dihydrochloride Tablets
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.’s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Industries
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL
NDC 47335-175-83
Levocetirizine Dihydrochloride Tablets
5 mg
For oral administration
Rx only
30 TABLETS
SUN PHARMA

Levocetirizine DihydrochlorideLevocetirizine Dihydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!
