Letomint
H&C 21 America Inc
H&C 21 America Inc
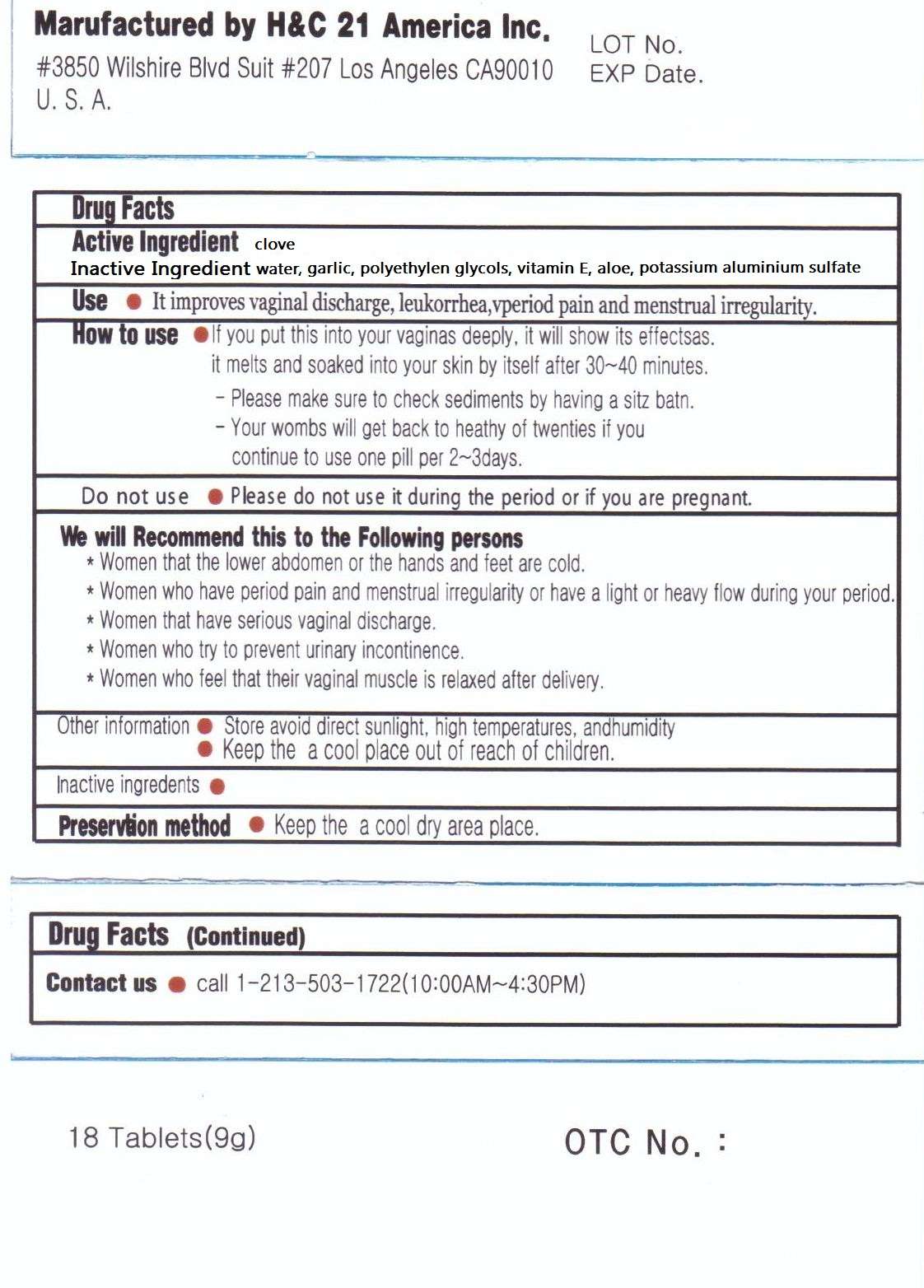
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Purpose
Uses
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:76255-2001 |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
CLOVE |
|
18 mg
|
Product Characteristics
|
|
Color
|
Size
|
Imprint Code
|
Shape
|
|
white (ivory white) |
10 mm |
3;hp;x |
OVAL |
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:76255-2001-1 |
18 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
|
|
2011-06-18 |
|
|
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!

