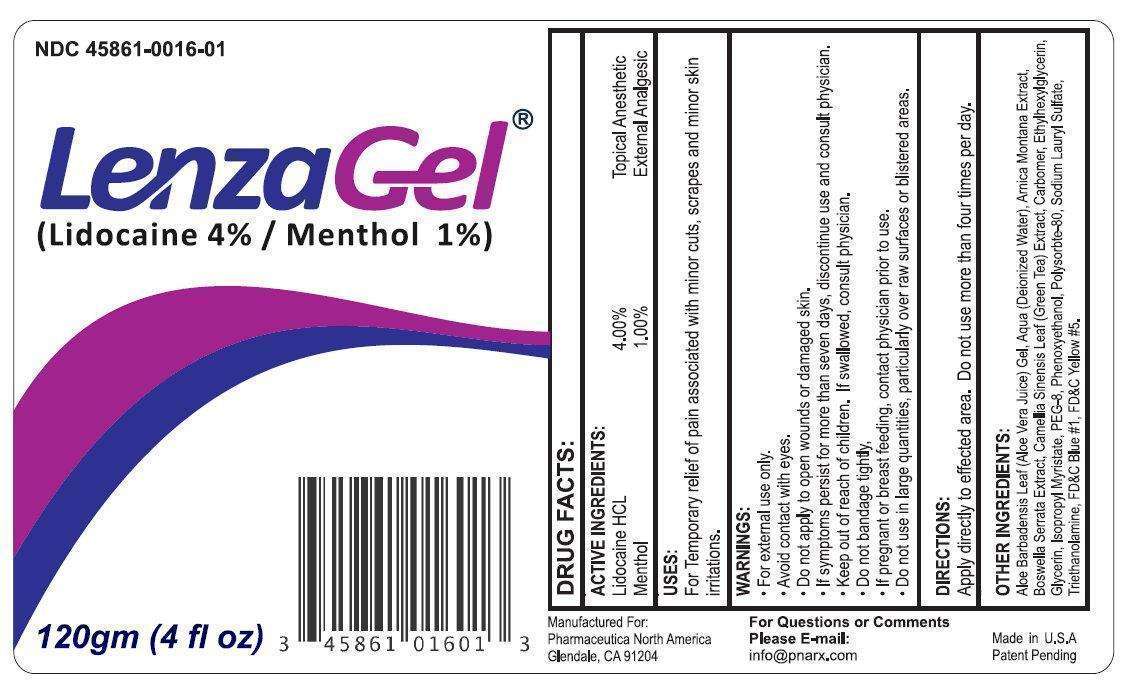
LenzaGel
Pharmaceutica North America, Inc.
LenzaGel
FULL PRESCRIBING INFORMATION: CONTENTS*
- LenzaGel
- Active Ingredients:
- Purpose
- LenzaGel Uses:
- Warnings
- Directions
- Other Ingredients:
- LenzaGel 120g (45861-016-01)
FULL PRESCRIBING INFORMATION
LenzaGel
Active Ingredients:
Lidocaine HCL 4.00%
Menthol 1.00%
Purpose
Topical Analgesic
External Analgesic
Uses:
For temporary relief of pain associated with minor cuts, scrapes and minor skin irritations.
Warnings
- For external use only
- Avoid contact with eyes
- Do not apply to open wounds or damaged skin.
- If symptoms persist for more than seven days, discontinue use and consult physician.
Keep out of reach of children.
If swallowed, consult physician.
- Do not bandage tightly
- If pregnant or breast feeding, contact physician prior to use.
- Do not use in large quantities, particularly over raw surfaces or blistered areas.
Directions
- Apply directly to effected area. Do not use more than four times per day.
Other Ingredients:
Aloe Barbadensis Leaf (Aloe Vera Juice) Gel, Aqua (Deionized Water), Arnica Montana Extract, Boswellia Serrata Extract, Camellia Sinensis Leaf (Green Tea) Extract, Carbomer, Ethylhexylglycerin, Glycerin, Isopropyl Myristate, PEG-8, Phenoxyethanol, Polysorbate-80, Sodium Lauryl Sulfate, Triethanolamine, FD C Blue 1, FD C Yellow 5.
LenzaGel 120g (45861-016-01)
LenzaGelLIDOCAINE HYDROCHLORIDE, MENTHOL GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!