LBel
EFFET PARFAIT
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- LBel Uses
- Warnings
- Directions
- LBel Other information
- Inactive ingredients
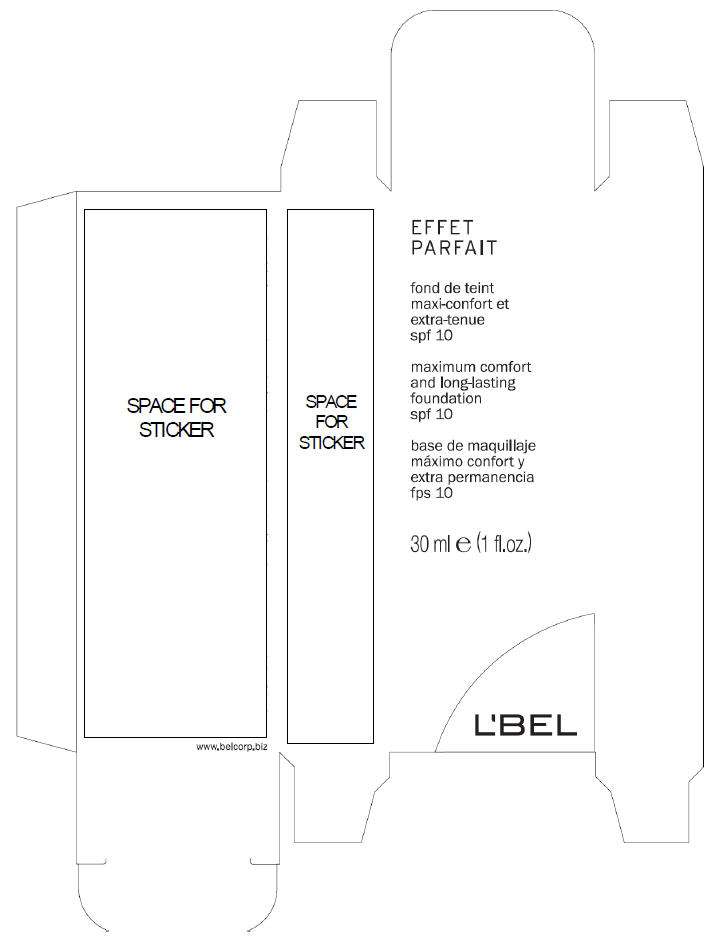
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Box
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredients
Zinc oxide 1.6 %
Purpose
sunscreen
LBel Uses
- Helps prevent sunburn
- Higher SPF gives more sunburn protection
- Provides minimal protection against sunburn
Warnings
For external use only
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash or irritation develops and lasts
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally before sun exposure and as needed
LBel Other information
- Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreen may reduce the risk of skin aging, skin cancer, and other harmful effects of the sun.
Inactive ingredients
aqua (water), cyclopentasiloxane, cyclomethicone, dimethicone, polymethyl methacrylate, peg/ppg-18/18 dimethicone, cyclohexasiloxane, methyl methacrylate crosspolymer, sodium chloride, bis-hydroxyethoxypropyl dimethicone, bis-peg 18 methyl ether dimethyl silane, peg/ppg-19/19 dimethicone, glycerin, oleth-5, phenyl trimethicone, hydrolyzed soy protein, propylene glycol, ci 77163 (bismuth oxychloride), silica silylate, ci 77019 (mica), trimethyilsiloxysilicate, methylparaben, triethoxycaprylylsilane, propylparaben, tocopheryl acetate, phenoxyethanol, parfum (fragrance), butylparaben, ethylparaben, silica, isobutylparaben. May contain: ci 77891 (titanium dioxide), ci 77492 (iron oxides), ci 77491 (iron oxides), ci 77499(iron oxides).
PRINCIPAL DISPLAY PANEL - 30 ml Bottle Box
EFFET
PARFAIT
maximum comfort
and long-lasting
foundation
spf 10
30 ml e (1 fl.oz.)
L'BEL
LBelZinc oxide LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||