LBel
L'BEL EFFET PARFAIT loose extra-fine portable powder SPF 15
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
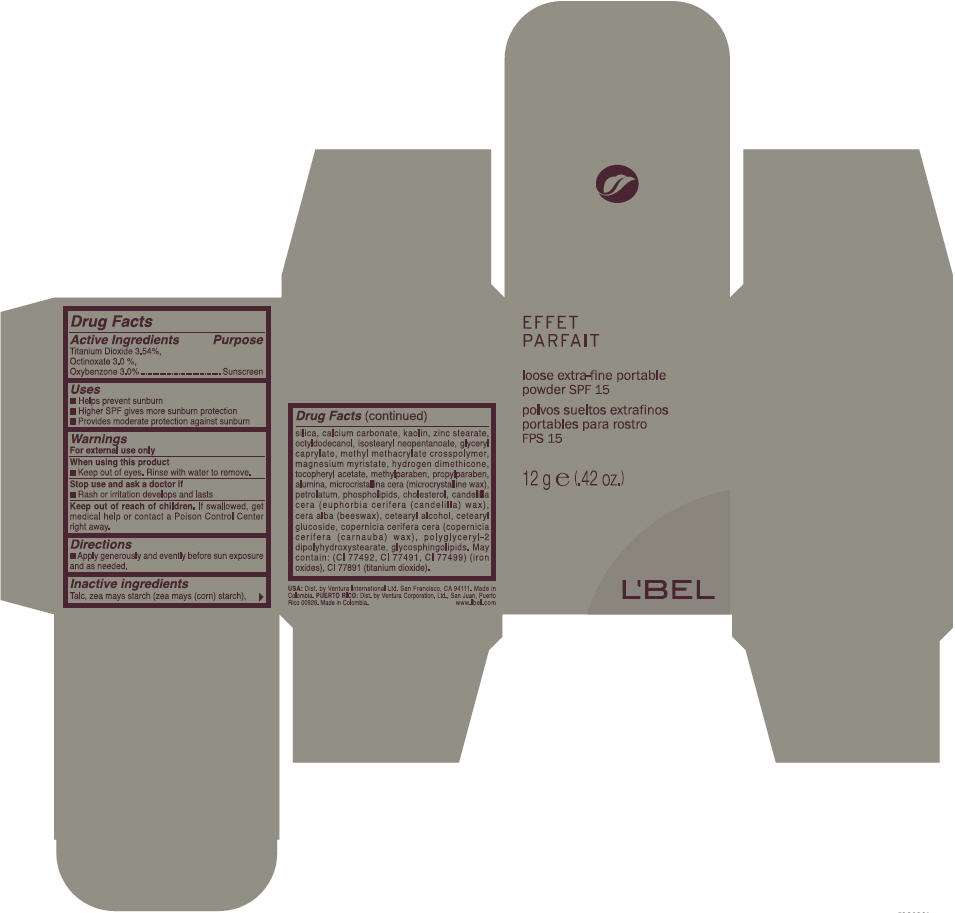
Drug Facts
Active ingredient
Purpose
| Active Ingredient | Purpose |
|---|---|
| Titanium Dioxide 3.5% | Sunscreen |
| Oxybenzone 3.0 % | Sunscreen |
| Octinoxate 3.0% | Sunscreen |
LBel Uses
- Helps prevent sunburn
- Higher SPF gives more sunburn protection
- Provides moderate protection against sunburn
Warnings
For external use only
When using this product
- Keep out of eyes. Rinse with water to remove
Stop use and ask a doctor if
- Rash or irritation develops and lasts
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply generously and evently before sun exposure and as needed
Inactive ingredients
Talc, Zea Mays Starch( Zea Mays (Corn) Starch), Titanium Dioxide, Silica, Benzophenone-3, Calcium Carbonate, Ethylhexyl Methoxycinnamate, Kaolin, Zinc Stearate, Octyldodecanol, Isostearyl Neopentanoate, Glyceryl Caprylate, Methyl Methacrylate Crosspolymer, Magnesium Myristate, Hydrogen Dimethicone, Tocopheryl Acetate, Methylparaben, Propylparaben, Alumina, Microcristallina Cera (Microcrystalline Wax), Petrolatum, Phospholipids, Cholesterol, Candelilla Cera (Euphorbia Cerifera (Candelilla Wax), Cera Alba (Beeswax), Cetearyl Alcohol, Cetearyl Glucoside, Copernicia Cerifera Cera (Copernicia Cerifera (Carnauba) Wax), Polyglyceryl-2 Dipolyhydroxystearate, Glycosphingolipids. May Contain: CI 77499, CI 77492, CI 77491 (Iron Oxides), CI 77891 (Titanium Dioxide).
Dist. by Ventura Corporation, Ltd., San Juan, Puerto Rico 00926.
PRINCIPAL DISPLAY PANEL - 12 g Bottle Carton
EFFET
PARFAIT
loose extra-fine portable
powder SPF 15
12 g e (.42 oz.)
L'BEL
LBelTITANIUM DIOXIDE, OXYBENZONE, and OCTINOXATE POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||