Ventura International LTD
L'BEL RENOVÂNCE JOUR
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
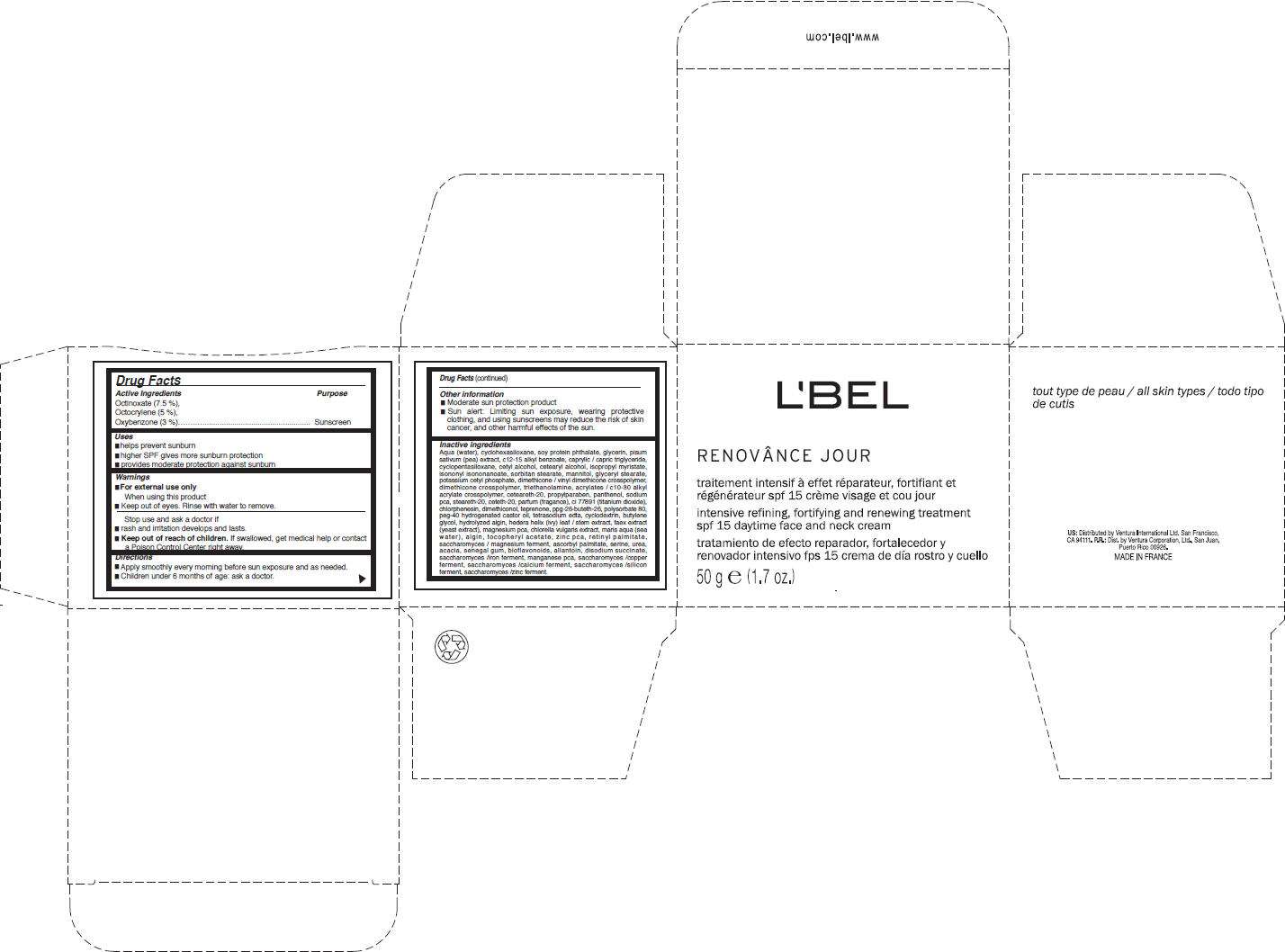
Drug Facts
Active Ingredients
Octinoxate (7.5 %), Octocrylene (5 %), Oxybenzone (3 %)
Purpose
Sunscreen
LBel Paris Uses
- Helps prevent sunburn
- Higher SPF gives more sunburn protection
- Provides moderate protection against sunburn
Warnings
When using this product
- keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
- rash and irritation develops and lasts.
-
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply smoothly every morning before sun exposure and as needed.
- Children under 6 months of age: ask a doctor.
LBel Paris Other information
- Moderate sun protection product.
- Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risk of skin cancer, and other harmful effects of the sun.
Inactive ingredients
Aqua (water), cyclohexasiloxane, soy protein phthalate, glycerin, pisum sativum (pea) extract, c12-15 alkyl benzoate, caprylic / capric triglyceride, cyclopentasiloxane, cetyl alcohol, cetearyl alcohol, isopropyl myristate, isononyl isononanoate, sorbitan stearate, mannitol, glyceryl stearate, potassium cetyl phosphate, dimethicone / vinyl dimethicone crosspolymer, dimethicone crosspolymer, triethanolamine, acrylates / c10-30 alkyl acrylate crosspolymer, ceteareth-20, propylparaben, panthenol, sodium pca, steareth-20, ceteth-20, parfum (fragance), ci 77891 (titanium dioxide), chlorphenesin, dimethiconol, teprenone, ppg-26-buteth-26, polysorbate 80, peg-40 hydrogenated castor oil, tetrasodium edta, cyclodextrin, butylene glycol, hydrolyzed algin, hedera helix (ivy) leaf / stem extract, faex extract (yeast extract), magnesium pca, chlorella vulgaris extract, maris aqua (sea water), algin, tocopheryl acetate, zinc pca, retinyl palmitate, saccharomyces / magnesium ferment, ascorbyl palmitate, serine, urea, acacia, senegal gum, bioflavonoids, allantoin, disodium succinate, saccharomyces /iron ferment, manganese pca, saccharomyces /copper ferment, saccharomyces /calcium ferment, saccharomyces /silicon ferment, saccharomyces /zinc ferment.
US: Distributed by Ventura International Ltd. San Francisco, CA 94111
PRINCIPAL DISPLAY PANEL - 50 g Jar Carton
L'BEL
RENOVÂNCE JOUR
intensive refining, fortifying and renewing treatment
spf 15 daytime face and neck cream
50 g e (1.7 oz.)
LBel Paris
Octinoxate, Octocrylene, and Oxybenzone CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:14783-038 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
3.75 g
|
|
OCTOCRYLENE Octocrylene |
|
2.5 g
|
|
OXYBENZONE OXYBENZONE |
|
1.5 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:14783-038-62 |
50 in 1 JAR |
|
|
|
2 |
NDC:14783-038-61 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-08-15 |
|
|
LBel Paris
Octinoxate, Octocrylene, and Oxybenzone CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:14783-028 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.375 g
|
|
OCTOCRYLENE Octocrylene |
|
0.25 g
|
|
OXYBENZONE OXYBENZONE |
|
0.15 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:14783-028-52 |
5 in 1 JAR |
|
|
|
2 |
NDC:14783-028-51 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-08-15 |
|
|
LBel Paris
Octinoxate, Octocrylene, and Oxybenzone CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:14783-018 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.075 g
|
|
OCTOCRYLENE Octocrylene |
|
0.05 g
|
|
OXYBENZONE OXYBENZONE |
|
0.03 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:14783-018-42 |
1 in 1 JAR |
|
|
|
2 |
NDC:14783-018-41 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-08-15 |
|
|
