Ventura International LTD
L'BEL FILLING EFFECT FOUNDATION SPF 10
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
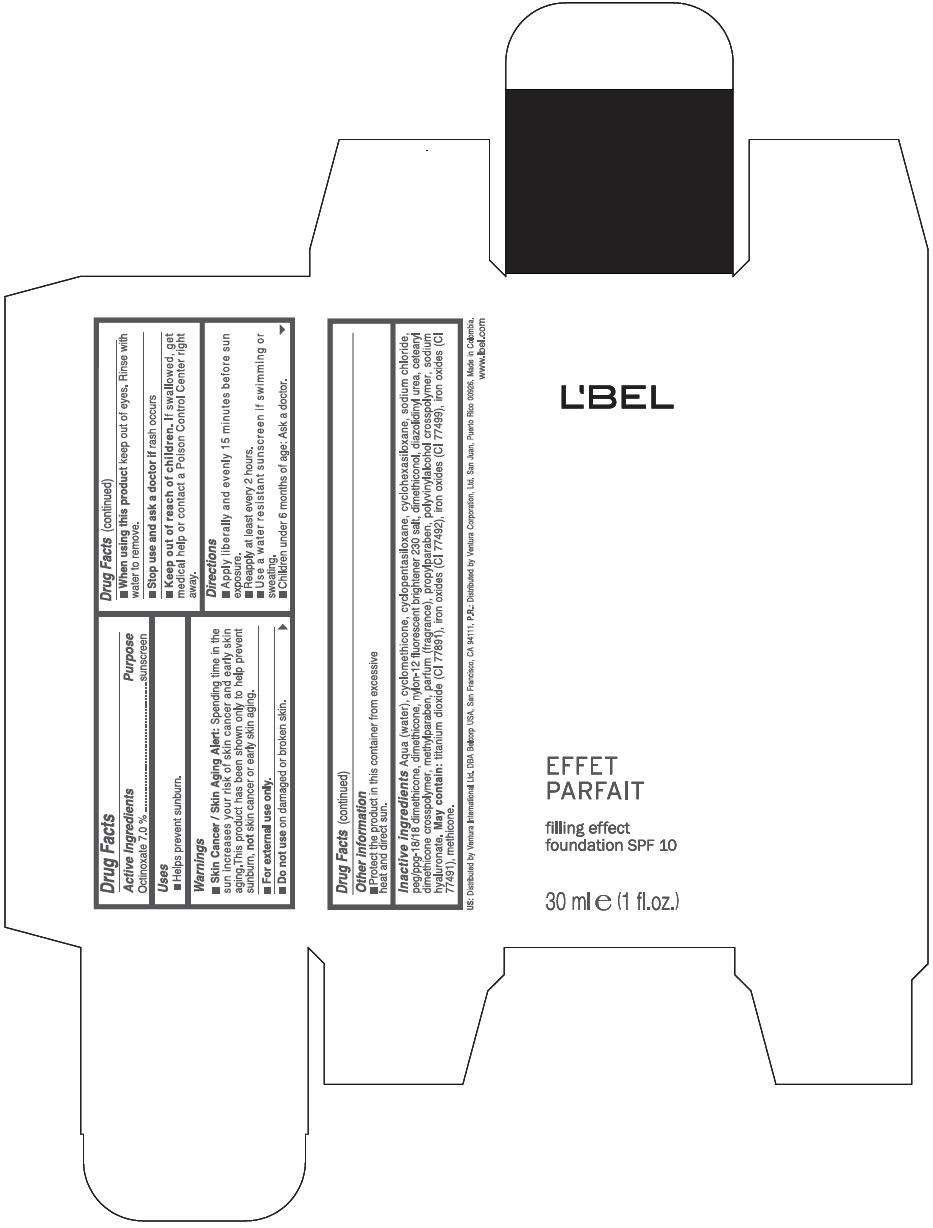
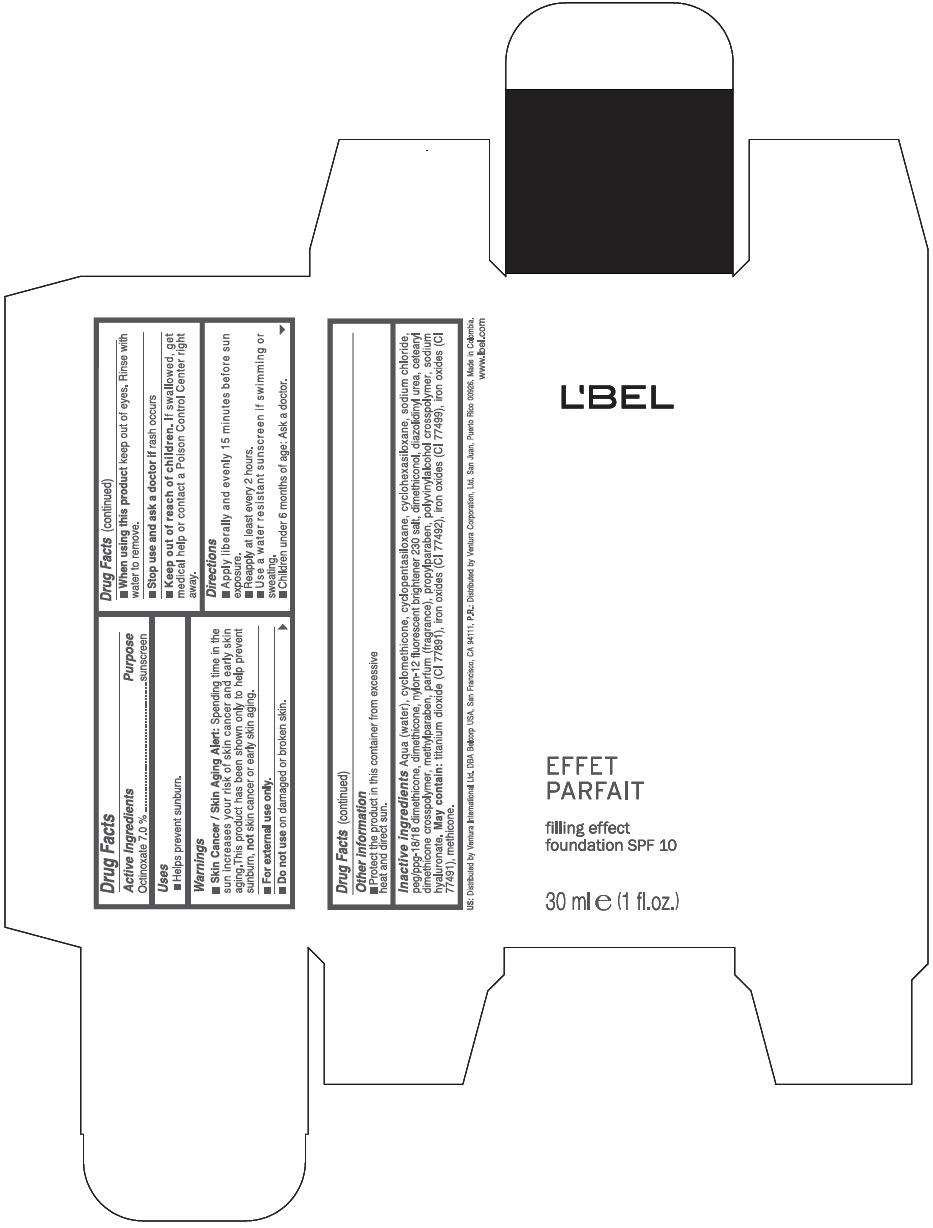
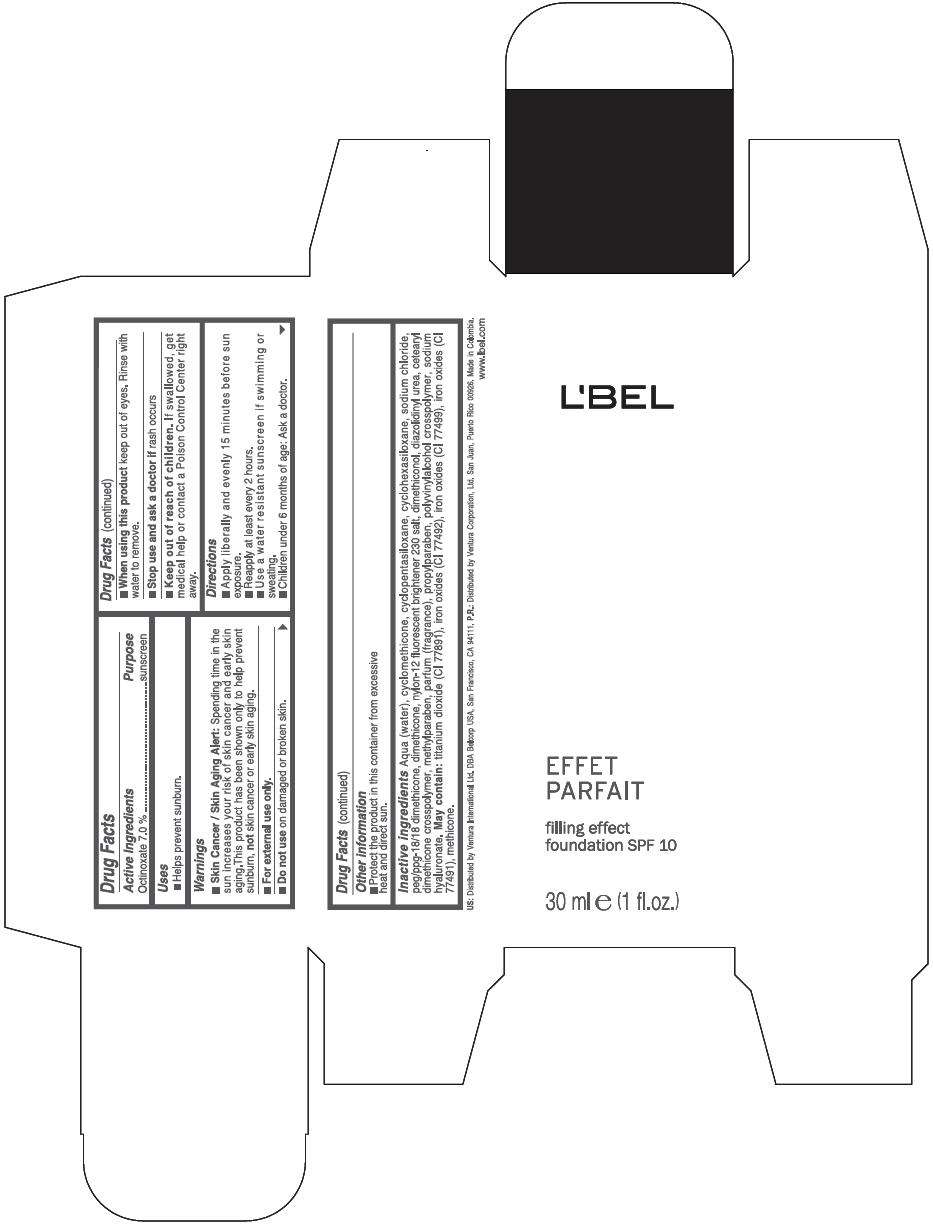
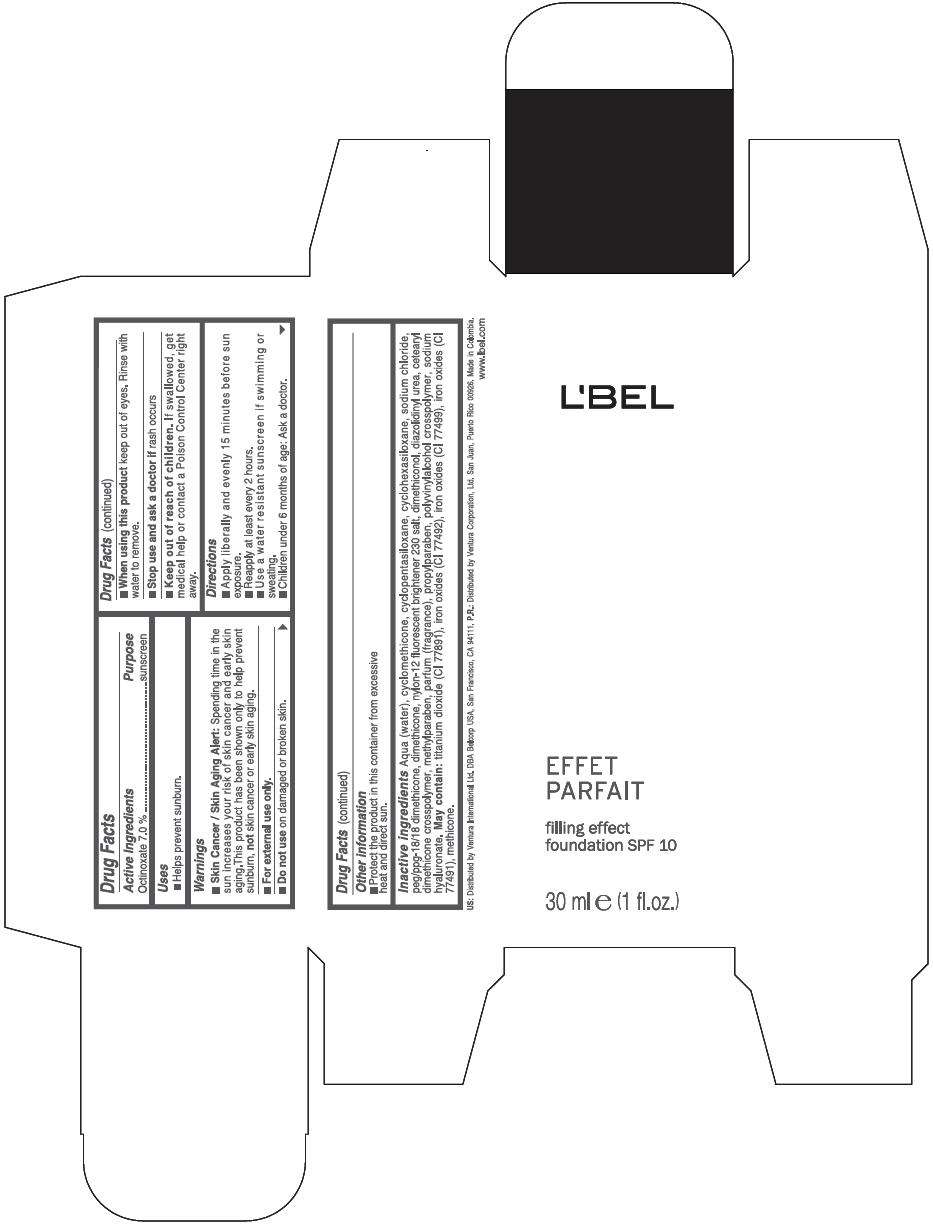
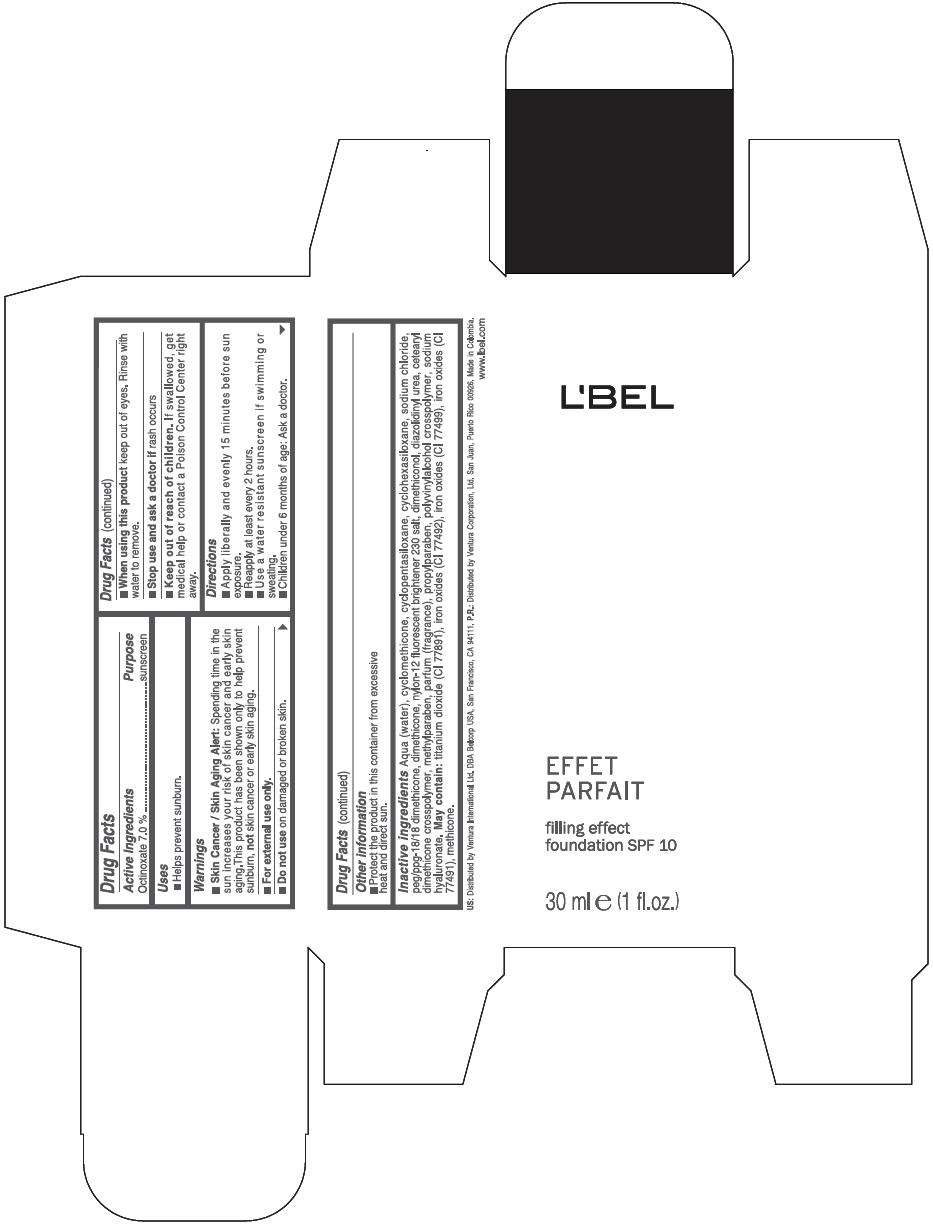
Drug Facts
Active Ingredients
Octinoxate 7 %
Purpose
Sunscreen
LBEL FILLING EFFECT FOUNDATION SPF 10 Uses
Warnings
-
Skin Cancer / Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging.This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
-
For external use only.
-
Do not use on damaged or broken skin.
-
When using this product keep out of eyes. Rinse with water to remove.
-
Stop use and ask a doctor if rash occurs
-
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally and evenly 15 minutes before sun exposure.
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating.
- Children under 6 months of age: Ask a doctor
LBEL FILLING EFFECT FOUNDATION SPF 10 Other information
- Protect the product in this container from excessive heat and direct sun.
Inactive ingredients
AQUA (WATER), CYCLOMETHICONE, CYCLOPENTASILOXANE, CYCLOHEXASILOXANE, SODIUM CHLORIDE, PEG/PPG-18/18 DIMETHICONE, DIMETHICONE, NYLON-12 FLUORESCENT BRIGHTENER 230 SALT, DIMETHICONOL, DIAZOLIDINYL UREA, CETEARYL DIMETHICONE CROSSPOLYMER, METHYLPARABEN, PARFUM (FRAGRANCE), PROPYLPARABEN, POLYVINYLALCOHOL CROSSPOLYMER, SODIUM HYALURONATE.
MAY CONTAIN
TITANIUM DIOXIDE (CI 77891), IRON OXIDES (CI 77492), IRON OXIDES (CI 77499), IRON OXIDES (CI 77491), METHICONE
Distributed by Ventura International Ltd. DBA Belcorp USA, San Francisco, CA 94111
PRINCIPAL DISPLAY PANEL - 30 ml Bottle Box - (Claire 1) - Beige
L'BEL
EFFET
PARFAIT
filling effect
foundation SPF 10
30 ml e (1 fl.oz.)
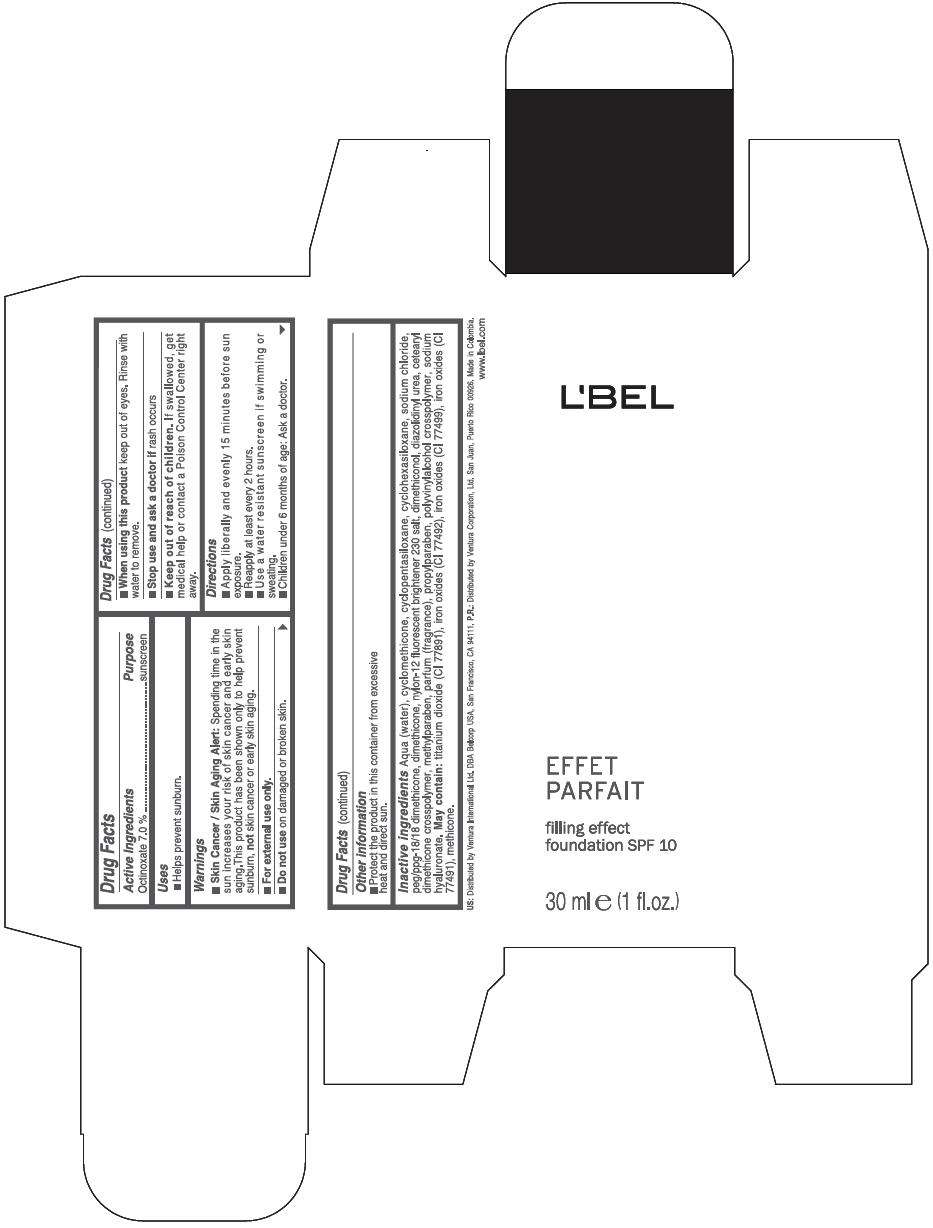
PRINCIPAL DISPLAY PANEL - 30 ml Bottle Box - (Claire 2) - Beige
L'BEL
EFFET
PARFAIT
filling effect
foundation SPF 10
30 ml e (1 fl.oz.)
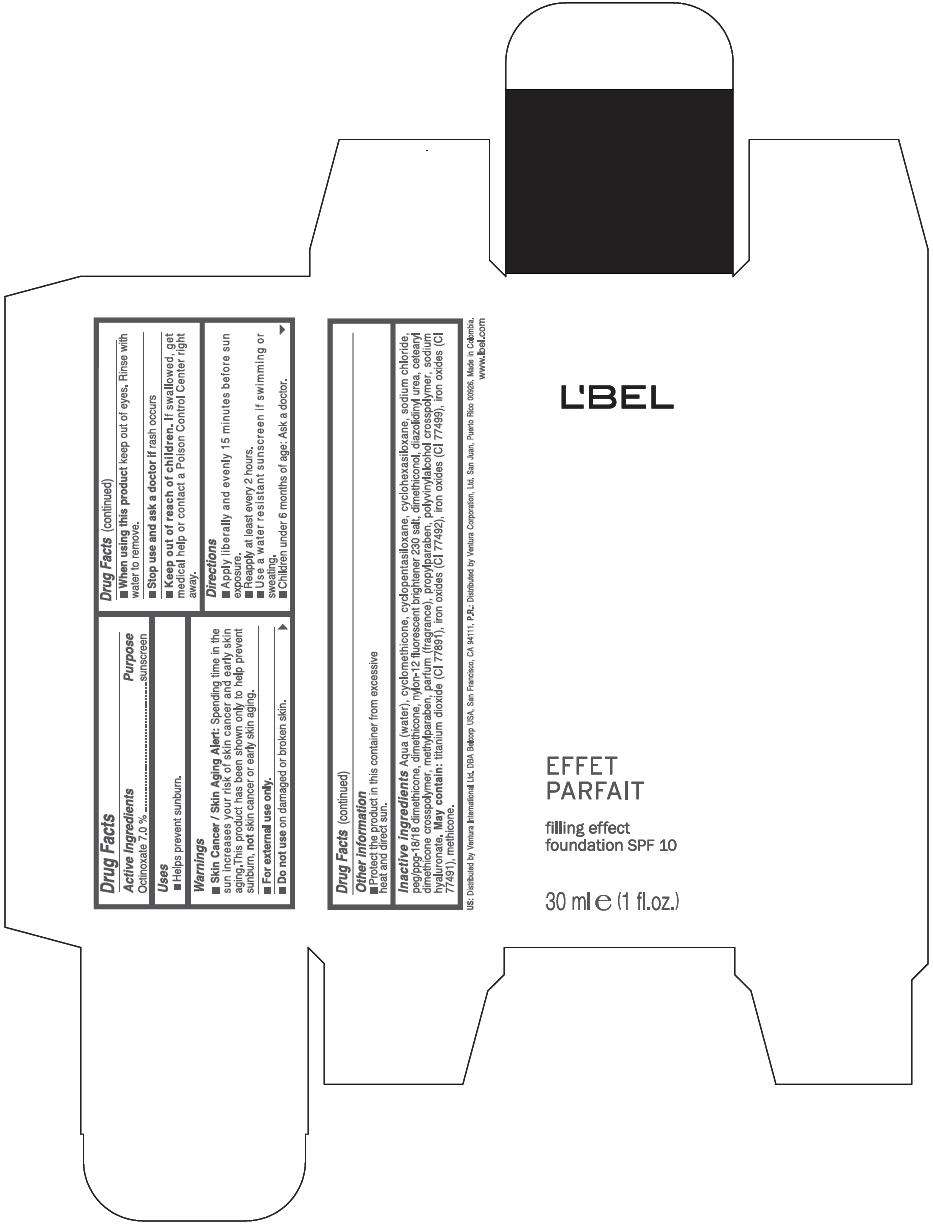
PRINCIPAL DISPLAY PANEL - 30 ml Bottle Box - (Claire 3) - Beige
L'BEL
EFFET
PARFAIT
filling effect
foundation SPF 10
30 ml e (1 fl.oz.)
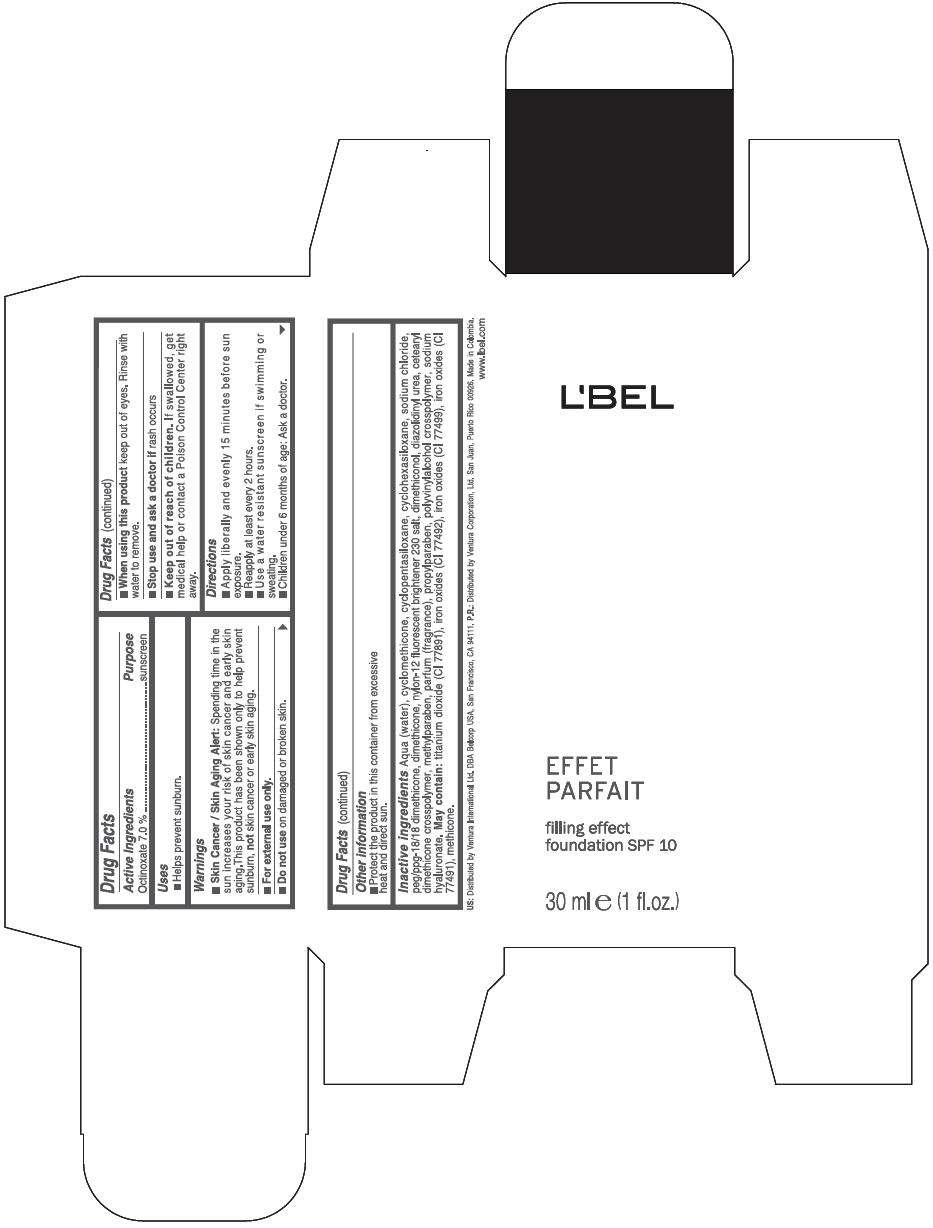
PRINCIPAL DISPLAY PANEL - 30 ml Bottle Box - (Claire 4) - Beige
L'BEL
EFFET
PARFAIT
filling effect
foundation SPF 10
30 ml e (1 fl.oz.)
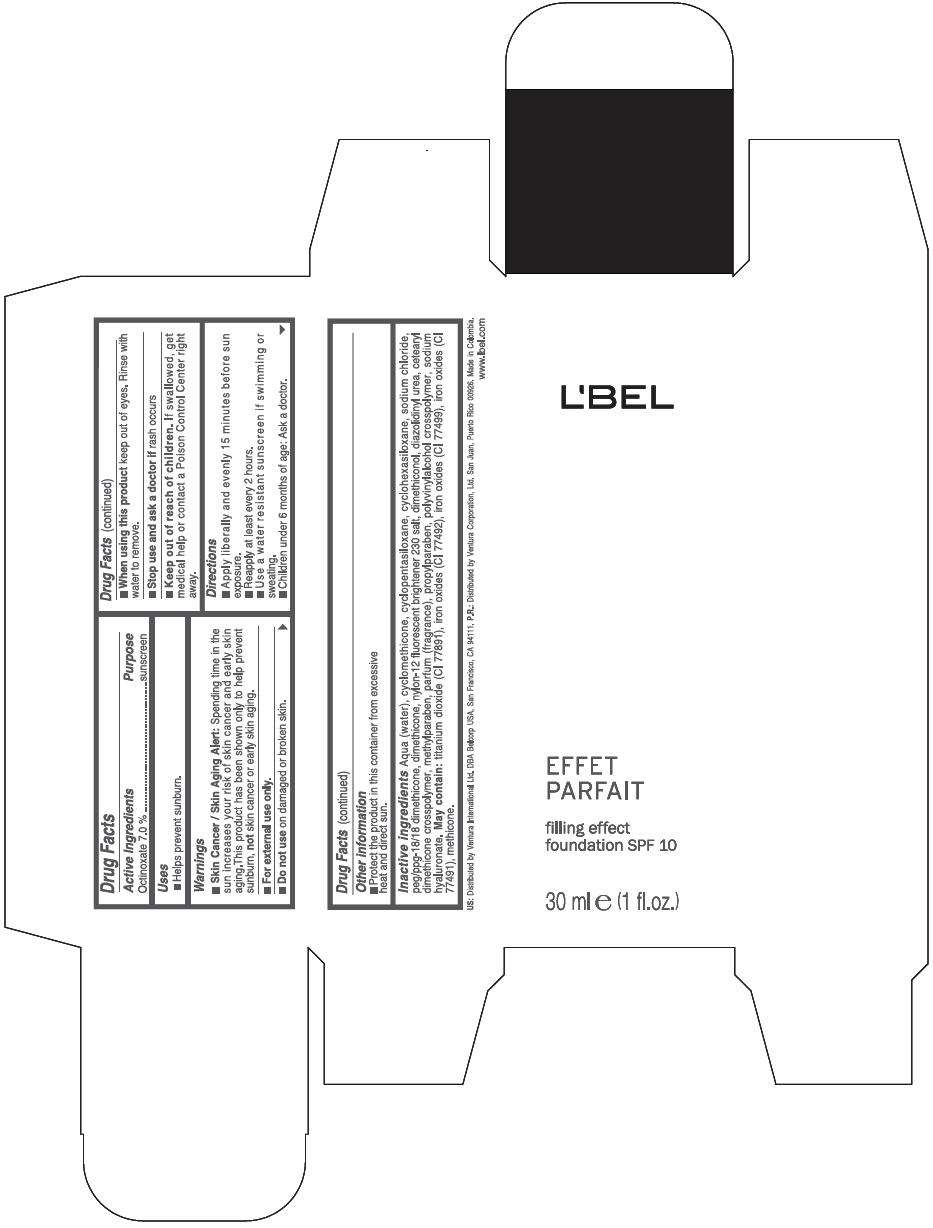
PRINCIPAL DISPLAY PANEL - 30 ml Bottle Box - (Medium 5) - Brown
L'BEL
EFFET
PARFAIT
filling effect
foundation SPF 10
30 ml e (1 fl.oz.)
PRINCIPAL DISPLAY PANEL - 30 ml Bottle Box - (Medium 6) - Brown
L'BEL
EFFET
PARFAIT
filling effect
foundation SPF 10
30 ml e (1 fl.oz.)
PRINCIPAL DISPLAY PANEL - 30 ml Bottle Box - (Medium 7) - Brown
L'BEL
EFFET
PARFAIT
filling effect
foundation SPF 10
30 ml e (1 fl.oz.)
PRINCIPAL DISPLAY PANEL - 30 ml Bottle Box - (Obscure 8) - Brown
L'BEL
EFFET
PARFAIT
filling effect
foundation SPF 10
30 ml e (1 fl.oz.)
PRINCIPAL DISPLAY PANEL - 30 ml Bottle Box - (Obscure 9) - Brown
L'BEL
EFFET
PARFAIT
filling effect
foundation SPF 10
30 ml e (1 fl.oz.)
LBEL FILLING EFFECT FOUNDATION SPF 10
Octinoxate LIQUID
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:14783-200 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.07 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:14783-200-01 |
30 in 1 BOTTLE |
|
|
|
2 |
NDC:14783-200-03 |
3 in 1 TUBE |
|
|
|
3 |
NDC:14783-200-04 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2013-12-16 |
|
|
LBEL FILLING EFFECT FOUNDATION SPF 10
Octinoxate LIQUID
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:14783-201 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.07 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:14783-201-05 |
30 in 1 BOTTLE |
|
|
|
2 |
NDC:14783-201-07 |
3 in 1 TUBE |
|
|
|
3 |
NDC:14783-201-08 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2013-12-16 |
|
|
LBEL FILLING EFFECT FOUNDATION SPF 10
Octinoxate LIQUID
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:14783-202 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.07 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:14783-202-09 |
30 in 1 BOTTLE |
|
|
|
2 |
NDC:14783-202-11 |
3 in 1 TUBE |
|
|
|
3 |
NDC:14783-202-12 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2013-12-16 |
|
|
LBEL FILLING EFFECT FOUNDATION SPF 10
Octinoxate LIQUID
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:14783-203 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.07 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:14783-203-13 |
30 in 1 BOTTLE |
|
|
|
2 |
NDC:14783-203-15 |
3 in 1 TUBE |
|
|
|
3 |
NDC:14783-203-16 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2013-12-16 |
|
|
LBEL FILLING EFFECT FOUNDATION SPF 10
Octinoxate LIQUID
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:14783-204 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.07 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:14783-204-17 |
30 in 1 BOTTLE |
|
|
|
2 |
NDC:14783-204-19 |
3 in 1 TUBE |
|
|
|
3 |
NDC:14783-204-20 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2013-12-16 |
|
|
LBEL FILLING EFFECT FOUNDATION SPF 10
Octinoxate LIQUID
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:14783-205 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.07 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:14783-205-21 |
30 in 1 BOTTLE |
|
|
|
2 |
NDC:14783-205-23 |
3 in 1 TUBE |
|
|
|
3 |
NDC:14783-205-24 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2013-12-16 |
|
|
LBEL FILLING EFFECT FOUNDATION SPF 10
Octinoxate LIQUID
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:14783-206 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.07 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:14783-206-25 |
30 in 1 BOTTLE |
|
|
|
2 |
NDC:14783-206-27 |
3 in 1 TUBE |
|
|
|
3 |
NDC:14783-206-28 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2013-12-16 |
|
|
LBEL FILLING EFFECT FOUNDATION SPF 10
Octinoxate LIQUID
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:14783-207 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.07 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:14783-207-29 |
30 in 1 BOTTLE |
|
|
|
2 |
NDC:14783-207-31 |
3 in 1 TUBE |
|
|
|
3 |
NDC:14783-207-32 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2013-12-16 |
|
|
LBEL FILLING EFFECT FOUNDATION SPF 10
Octinoxate LIQUID
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:14783-208 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.07 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:14783-208-33 |
30 in 1 BOTTLE |
|
|
|
2 |
NDC:14783-208-35 |
3 in 1 TUBE |
|
|
|
3 |
NDC:14783-208-36 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2013-12-16 |
|
|








