LBEL EFFET PARFAIT Spots Reducing Effect Foundation SPF 18 - CLAIRE 3
L'BEL EFFET PARFAIT Spots Reducing Effect Foundation SPF 18 - CLAIRE 3
FULL PRESCRIBING INFORMATION: CONTENTS*
- L'BEL EFFET PARFAIT Spots Reducing Effect Foundation SPF 18 - CLAIRE 3
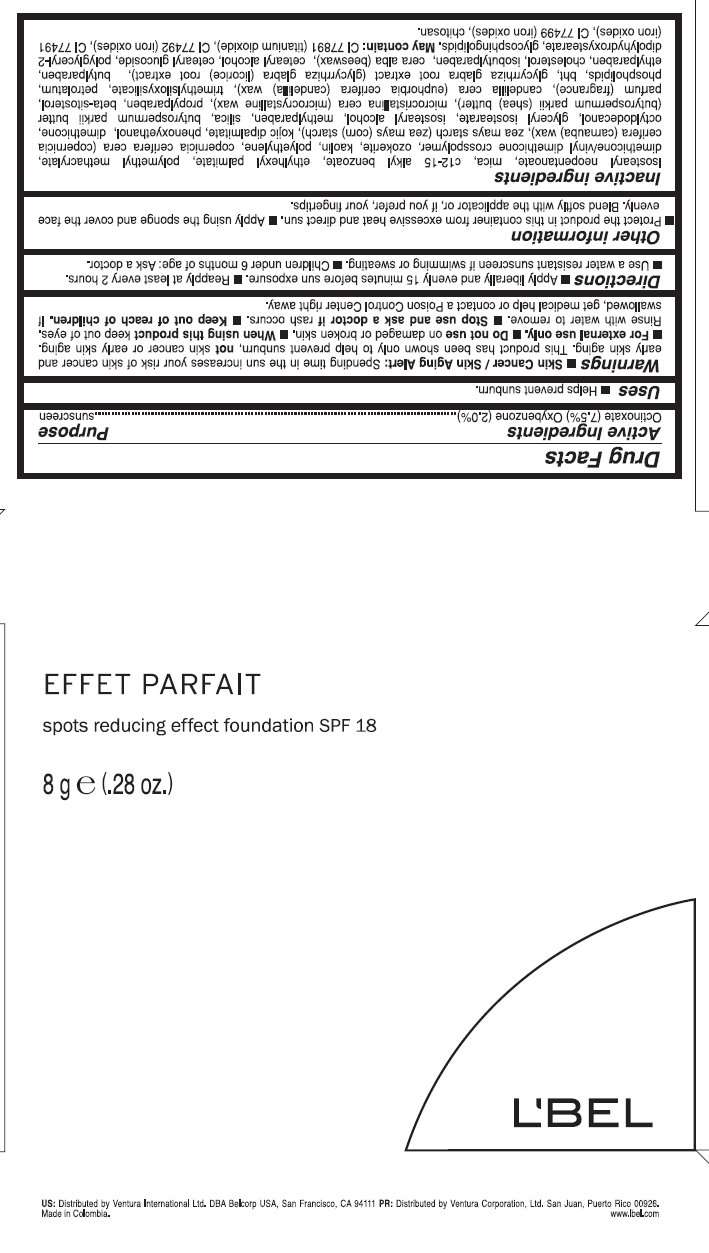
- Active Ingredients
- LBEL EFFET PARFAIT Spots Reducing Effect Foundation SPF 18 - CLAIRE 3 Uses
- Warnings
- Directions
- Other Information
- Inactive ingredients
- Purpose
- EFFET PARFAIT
- L'BEL EFFET PARFAIT Spots Reducing Effect Foundation SPF 18 - CLAIRE 3 8g
FULL PRESCRIBING INFORMATION
L'BEL EFFET PARFAIT Spots Reducing Effect Foundation SPF 18 - CLAIRE 3
Active Ingredients
Octinoxate (7.5%), Oxybenzone (2.0%)
LBEL EFFET PARFAIT Spots Reducing Effect Foundation SPF 18 - CLAIRE 3 Uses
- Helps prevent sunburn.
Warnings
- Skin Cancer / Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
- For external use only.
Do not use
on damaged or broken skin.
When using this product
keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor
if rash occurs.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally and evenly 15 minutes before sun exposure.
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating.
- Children under 6 months of age: Ask a doctor.
Other Information
- Protect the product in this container from excessive heat and direct sun.
- Apply using the sponge and cover the face evenly. Blend softly with the applicator or, if you prefer, your fingertips.
Inactive ingredients
Isostearyl Neopentanoate, Mica, C12-15 Alkyl Benzoate, Ethylhexyl Palmitate, Polymethyl Methacrylate, Dimethicone/Vinyl Dimethicone Crosspolymer, Ozokerite, Kaolin, Polyethylene, Copernicia Cerifera Cera (Copernicia Cerifera (Carnauba) Wax), Zea Mays Starch (Zea Mays (Corn) Starch), Kojic Dipalmitate, Phenoxyethanol, Dimethicone, Octyldodecanol, Glyceryl Isostearate, Isostearyl Alcohol, Methylparaben, Silica, Butyrospermum Parkii Butter (Butyrospermum Parkii (Shea) Butter), Microcristallina Cera (Microcrystalline Wax), Propylparaben, Beta-Sitosterol, Parfum (Fragrance), Candelilla Cera (Euphorbia Cerifera (Candelilla) Wax), Trimethylsiloxysilicate, Petrolatum, Phospholipids, Bht, Glycyrrhiza Glabra Root Extract (Glycyrrhiza Glabra (Licorice) Root Extract), Butylparaben, Ethylparaben, Cholesterol, Isobutylparaben, Cera Alba (Beeswax), Cetearyl Alcohol, Cetearyl Glucoside, Polyglyceryl-2 Dipolyhydroxystearate, Glycosphingolipids.
May Contain: Ci 77891 (Titanium Dioxide), Ci 77492 (Iron Oxides), Ci 77491 (Iron Oxides), Ci 77499 (Iron Oxides), Chitosan
Purpose
Sunscreen
EFFET PARFAIT
Facial Perfecting LineUSES:
WARNINGS
DIRECTIONS:
Other Information:
Step 1:
Step 2:
Step 3:
Step 4:
L'BEL EFFET PARFAIT Spots Reducing Effect Foundation SPF 18 - CLAIRE 3 8g


LBEL EFFET PARFAIT Spots Reducing Effect Foundation SPF 18 - CLAIRE 3OCTINOXATE, OXYBENZONE EMULSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||