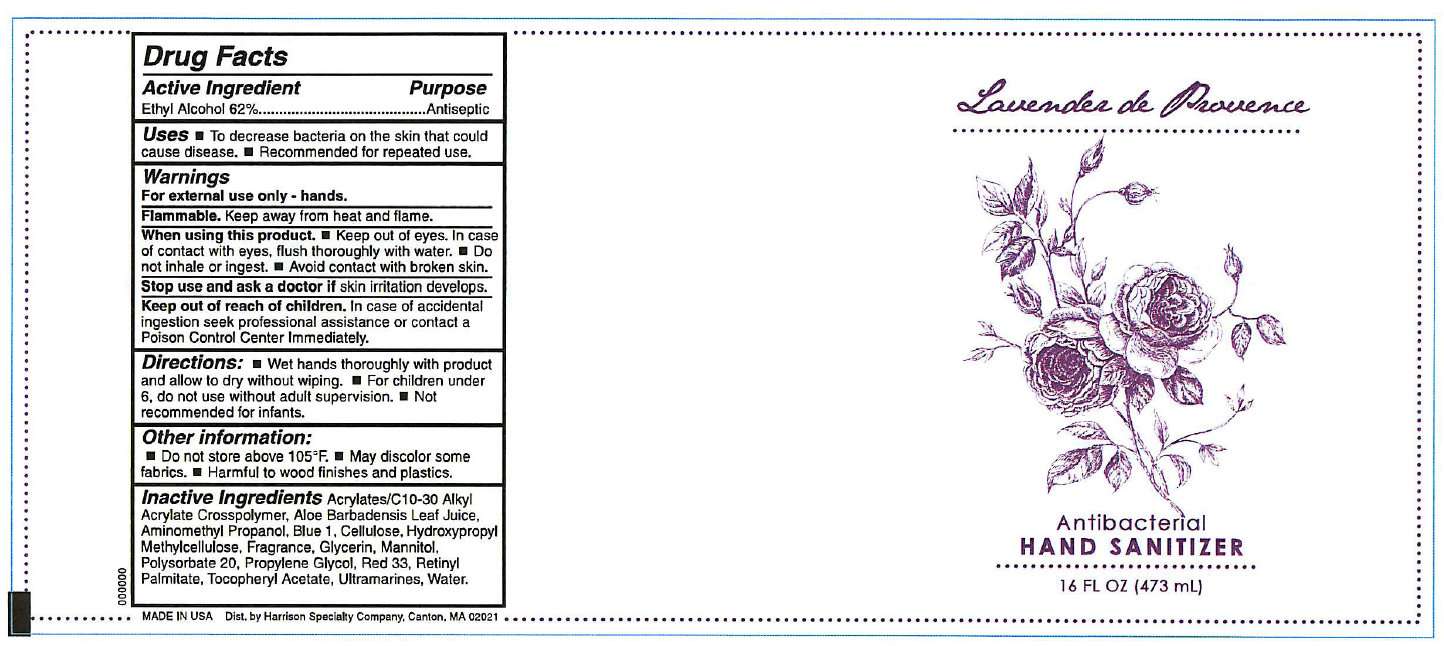
Lavender de Provence Antibacterial Hand Sanitizer
Harrison Specialty Co., Inc.
Harrison Specialty Co., Inc.
Lavender de Provence Antibacterial Hand Sanitizer
FULL PRESCRIBING INFORMATION: CONTENTS*
- DRUG FACTS
- ACTIVE INGREDIENT
- PURPOSE
- USES
- WARNINGS
- DIRECTIONS:
- OTHER INFORMATION:
- INACTIVE INGREDIENTS
- Lavender de Provence Antibacterial Hand Sanitizer 16 FL OZ / 473ml
FULL PRESCRIBING INFORMATION
DRUG FACTS
ACTIVE INGREDIENT
Ethyl Alcohol 62%
PURPOSE
Antiseptic
USES
- To decrease bacteria on the skin that could cause disease.
- Recommended for repeated use.
WARNINGS
For external use only - hands.
Flammable.
Keep away from heat and flame.
When using this product.
- Keep out of eyes. In case of contact with eyes, flush thoroughly with water.
- Do not inhale or ingest.
- Avoid contact with broken skin.
Stop use and ask a doctor if
skin irritation develops.
Keep out of reach of children.
In case of accidental ingestion seek professional assistance or contact a Poison Control Center immediately.
DIRECTIONS:
- Wet hands thoroughly with product and allow to dry without wiping.
- For children under 6, do not use without adult supervision.
- Not recommended for infants.
OTHER INFORMATION:
- Do not store above 105 degrees F.
- May discolor some fabrics.
- Harmful to wood finishes and plastics.
INACTIVE INGREDIENTS
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, Aminomethyl Propanol, Blue 1, Cellulose, Hydroxypropyl Methylcellulose, Fragrance, Glycerin, Mannitol, Polysorbate 20, Propylene Glycol, Red 33, Retinyl Palmitate, Tocopheryl Acetate, Ultramarines, Water.
MADE IN USA
Dist. by Harrison Specialty Company, Canton, MA 02021
Lavender de Provence Antibacterial Hand Sanitizer 16 FL OZ / 473ml
Lavender de Provence Antibacterial Hand SanitizerALCOHOL LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||