AMOREPACIFIC
LANEIGE SATIN FINISH TWIN PACT SPF 25 / PA++
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredients
| TITANIUM DIOXIDE |
8 % |
| ETHYLHEXYL METHOXYCINNAMATE |
6 % |
| ZINC OXIDE |
2.94 % |
WARNINGS
For external use only.
When using this product, Keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash and irritation develops and lasts.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Use after makeup base or foundation.
- Tip out the contents with your finger and place on the parts where you want to cover a little bit at a time. Tap it on the part where you want to camouflage.
Inactive Ingredients
TALC, POLYMETHYL METHACRYLATE, DIISOSTEARYL MALATE, ALUMINA, DIMETHICONE, TRIETHOXYCAPRYLYLSILANE, METHICONE, MAGNESIUM STEARATE, ALUMINUM STEARATE, ACRYLATES/TRIDECYL ACRYLATE/TRIETHOXYSILYLPROPYL METHACRYLATE/DIMETHICONE METHACRYLATE COPOLYMER, SYNTHETIC FLUORPHLOGOPITE, WATER, BUTYLENE GLYCOL, BEESWAX, POLYGLYCERYL-3 METHYLGLUCOSE DISTEARATE, INULIN LAURYL CARBAMATE, HYDROXYPROPYL BISPALMITAMIDE MEA, METHYLPARABEN, BUTYLPARABEN, FRAGRANCE, MICA, TITANIUM DIOXIDE (CI 77891), IRON OXIDES (CI 77491, CI 77492, CI 77499), ULTRAMARINES (CI 77007)
13 g / 0.5 OZ. e
AMOREPACIFIC US INC.
270 Sylvan Ave. #255
Englewood Cliffs, NJ07632
MADE IN KOREA
(5240)
PRINCIPAL DISPLAY PANEL - 13 g Carton Label, No. 13
LANEÍGE
SATIN FINISH TWIN PACT
SPF25 PA++

PRINCIPAL DISPLAY PANEL - 13 g Carton Label, No. 21
LANEÍGE
SATIN FINISH TWIN PACT
SPF25 PA++
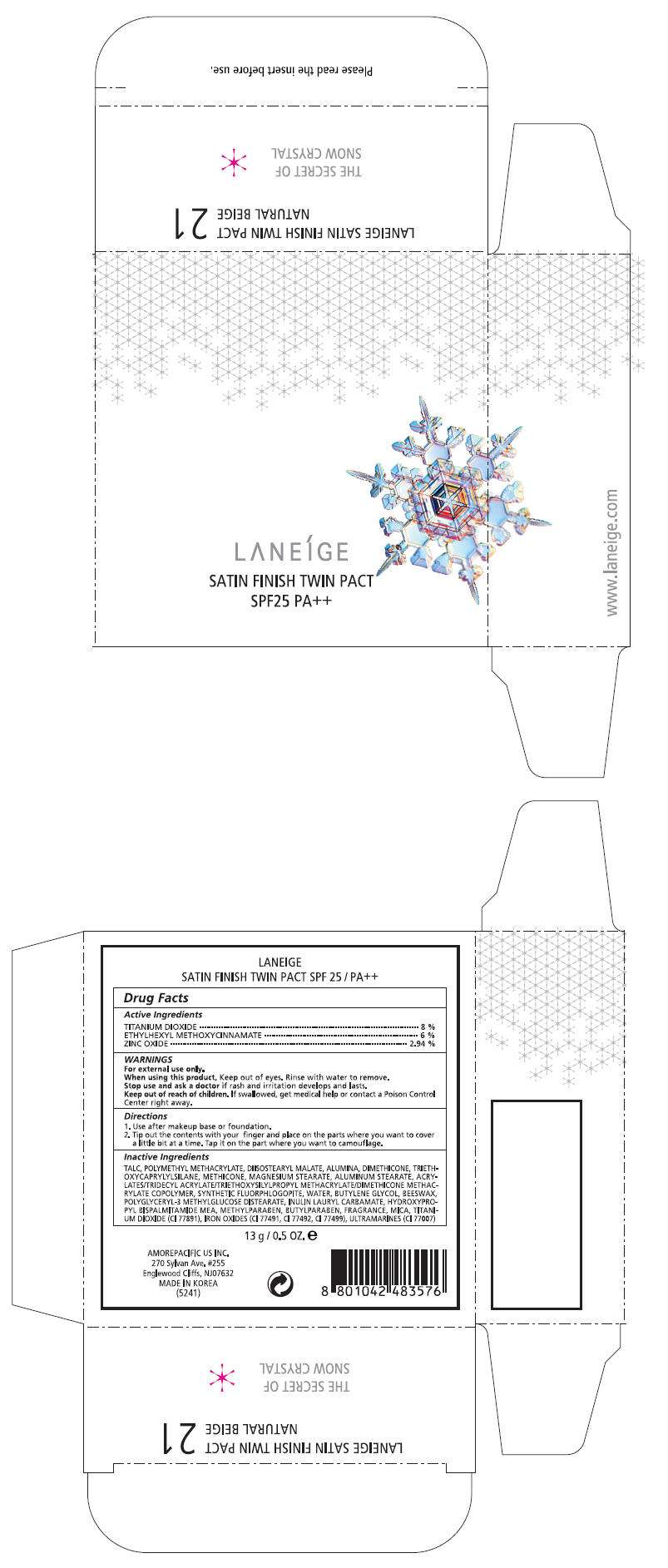
PRINCIPAL DISPLAY PANEL - 13 g Carton Label, No. 23
LANEÍGE
SATIN FINISH TWIN PACT
SPF25 PA++
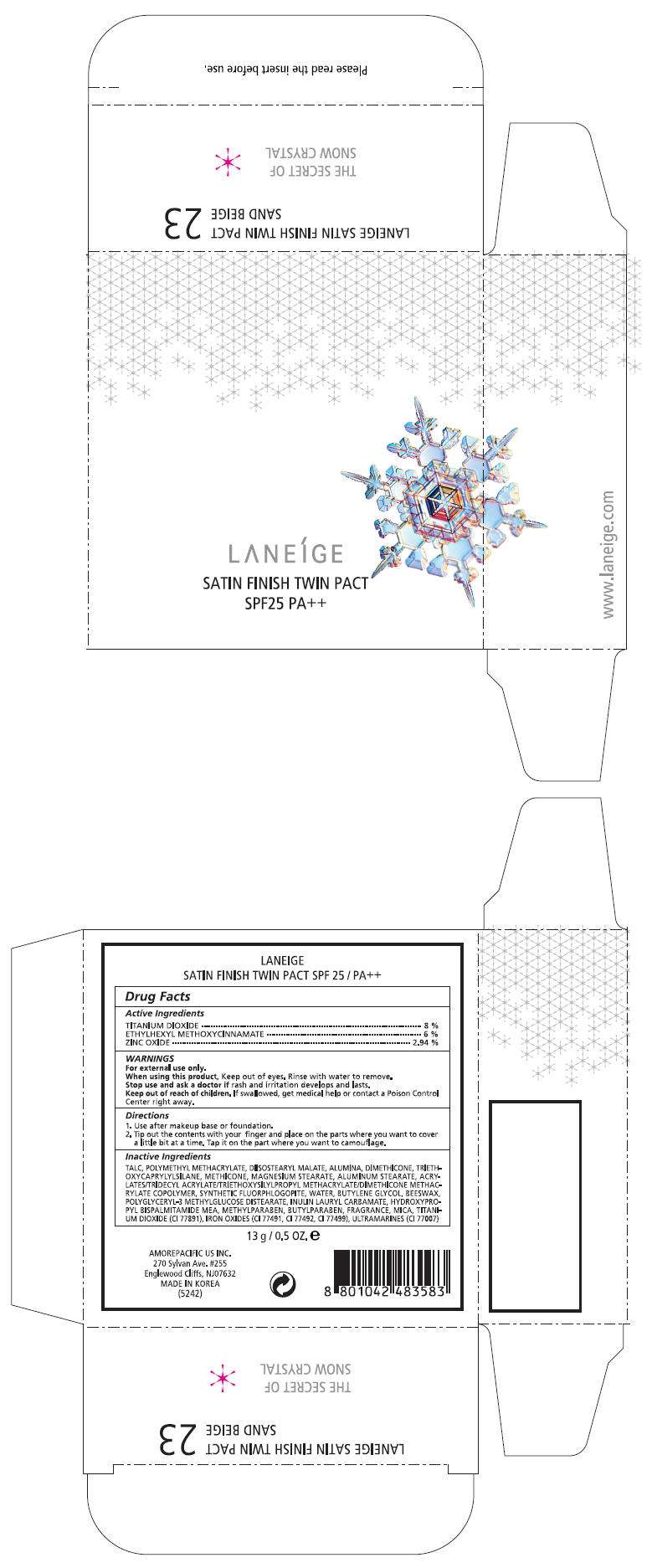
PRINCIPAL DISPLAY PANEL - 13 g Carton Label, No. 25
LANEÍGE
SATIN FINISH TWIN PACT
SPF25 PA++
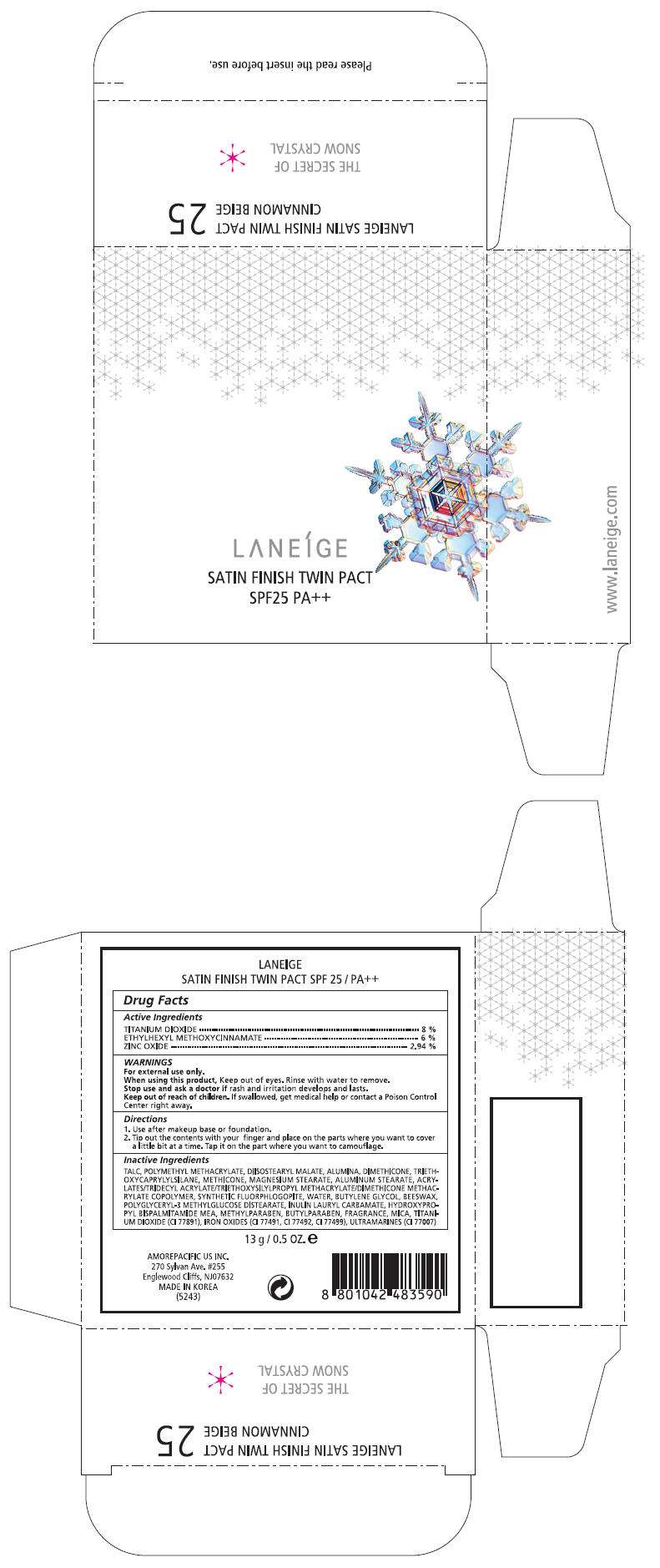
PRINCIPAL DISPLAY PANEL - 13 g Carton Label, No. 31
LANEÍGE
SATIN FINISH TWIN PACT
SPF25 PA++
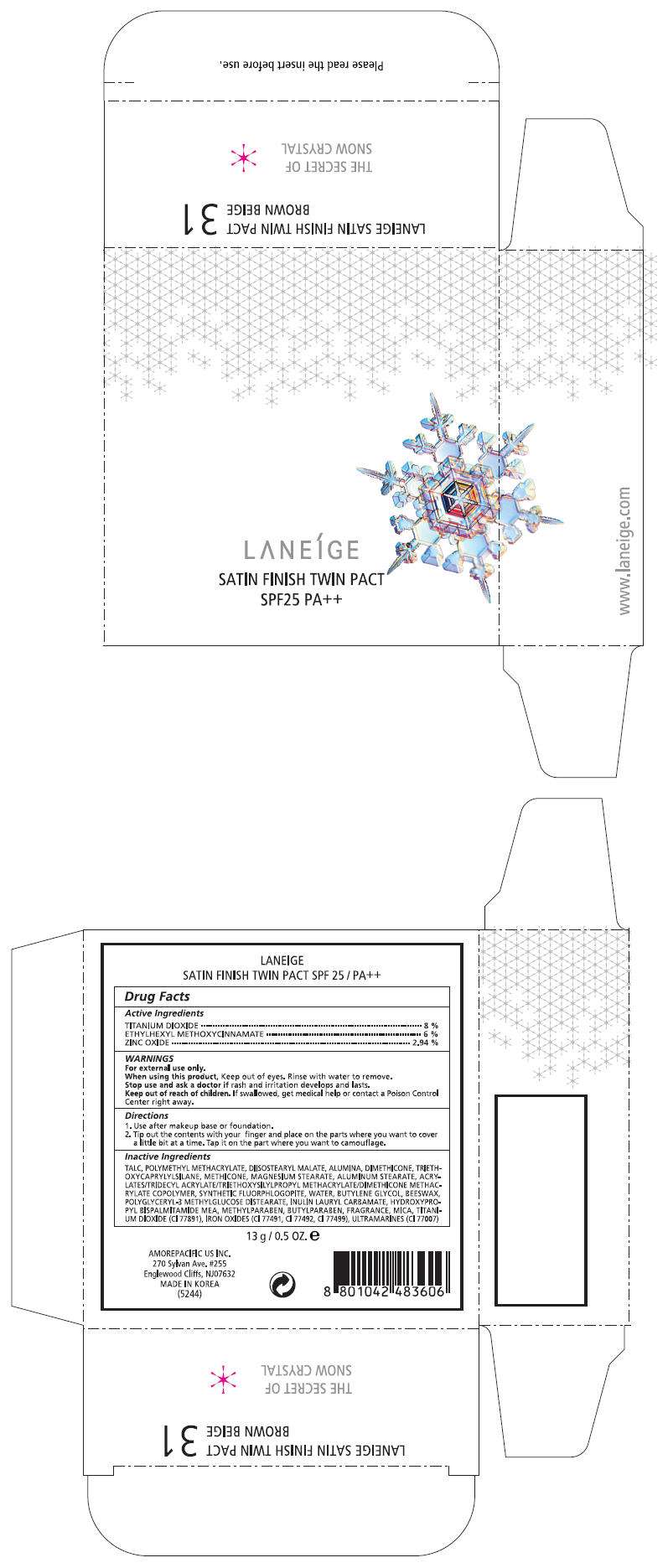
LANEIGE SATIN FINISH TWIN PACT NO. 13
TITANIUM DIOXIDE, OCTINOXATE, and ZINC OXIDE POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:43419-863 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:43419-863-40 |
13 in 1 CONTAINER |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-12-01 |
|
|
LANEIGE SATIN FINISH TWIN PACT NO. 21
TITANIUM DIOXIDE, OCTINOXATE, and ZINC OXIDE POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:43419-864 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:43419-864-41 |
13 in 1 CONTAINER |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-12-01 |
|
|
LANEIGE SATIN FINISH TWIN PACT NO. 23
TITANIUM DIOXIDE, OCTINOXATE, and ZINC OXIDE POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:43419-865 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:43419-865-42 |
13 in 1 CONTAINER |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-12-01 |
|
|
LANEIGE SATIN FINISH TWIN PACT NO. 25
TITANIUM DIOXIDE, OCTINOXATE, and ZINC OXIDE POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:43419-866 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:43419-866-43 |
13 in 1 CONTAINER |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-12-01 |
|
|
LANEIGE SATIN FINISH TWIN PACT NO. 31
TITANIUM DIOXIDE, OCTINOXATE, and ZINC OXIDE POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:43419-867 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:43419-867-44 |
13 in 1 CONTAINER |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-12-01 |
|
|




