LANEIGE LIP TREATMENT
LANEIGE LIP TREATMENT SPF 17
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENTS
- PURPOSE
- USE
- WARNINGS
- DIRECTIONS
- INACTIVE INGREDIENTS
- OTHER INFORMATION
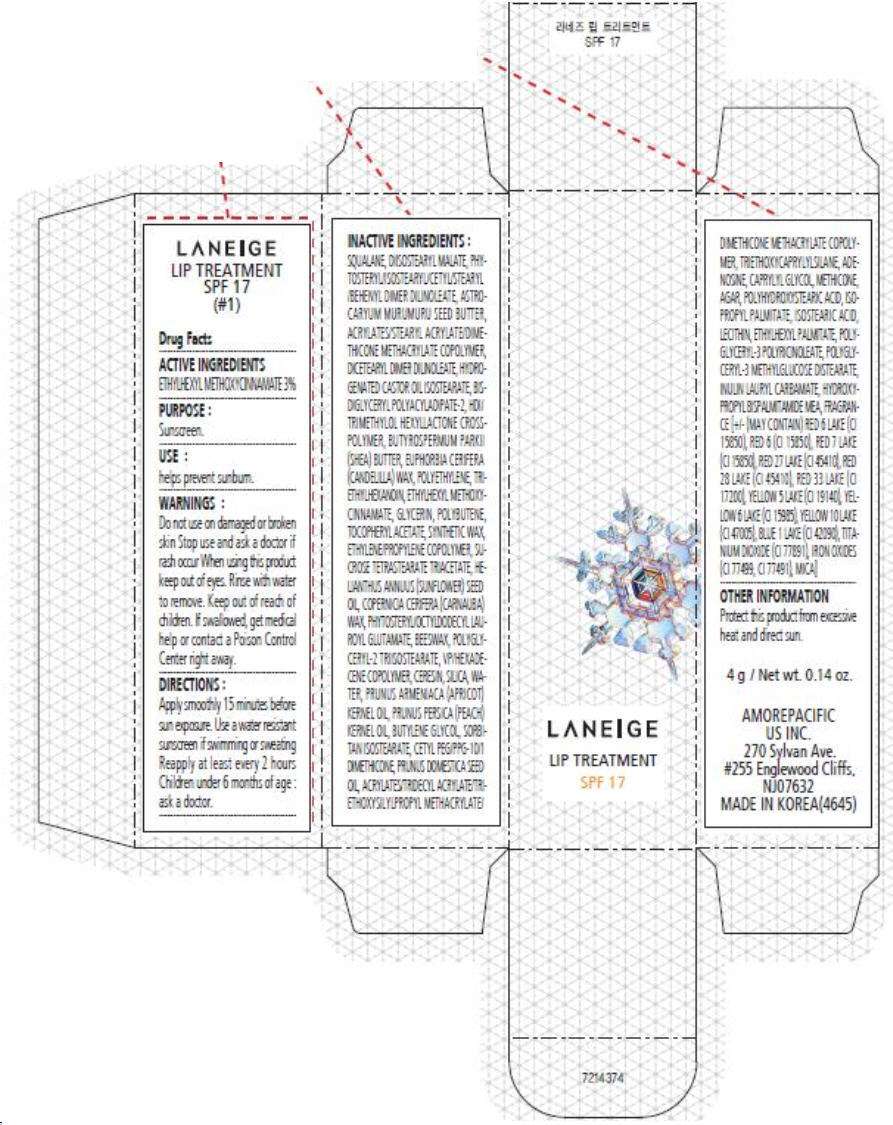
- PRINCIPAL DISPLAY PANEL - 4 g Carton (#1)
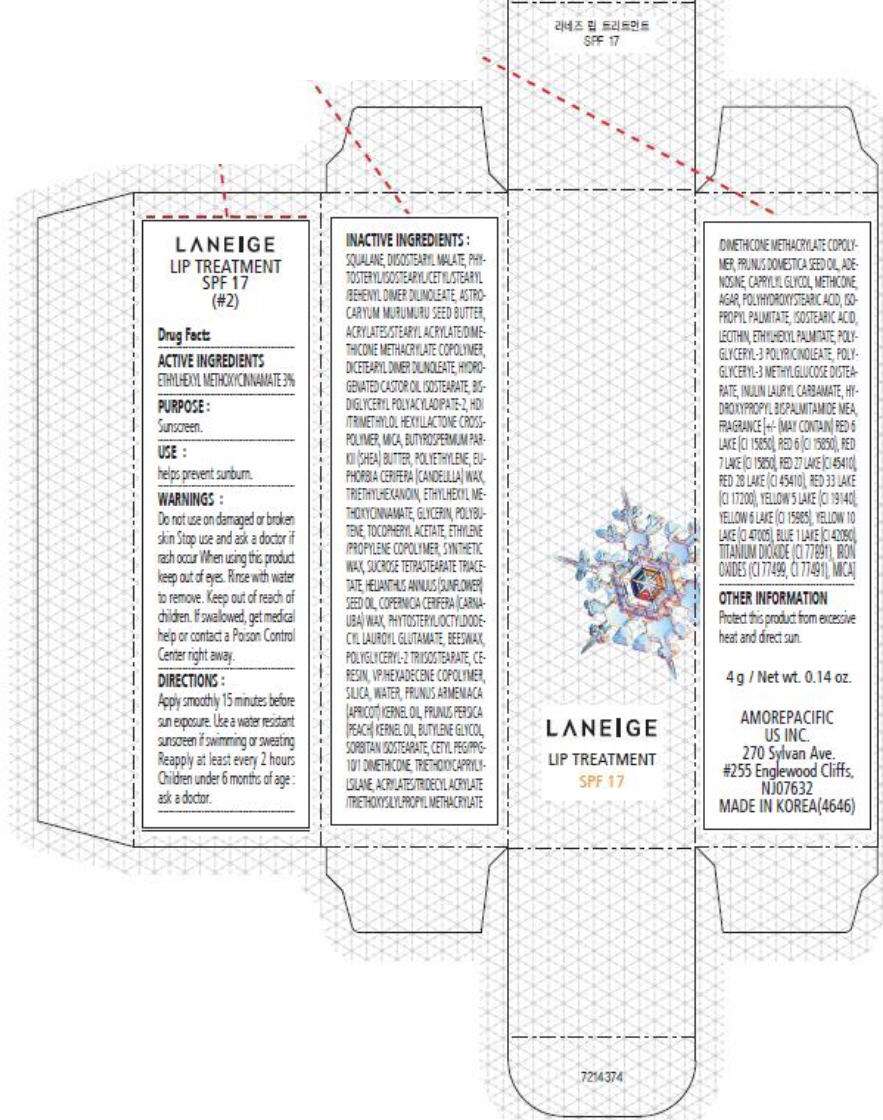
- PRINCIPAL DISPLAY PANEL - 4 g Carton (#2)
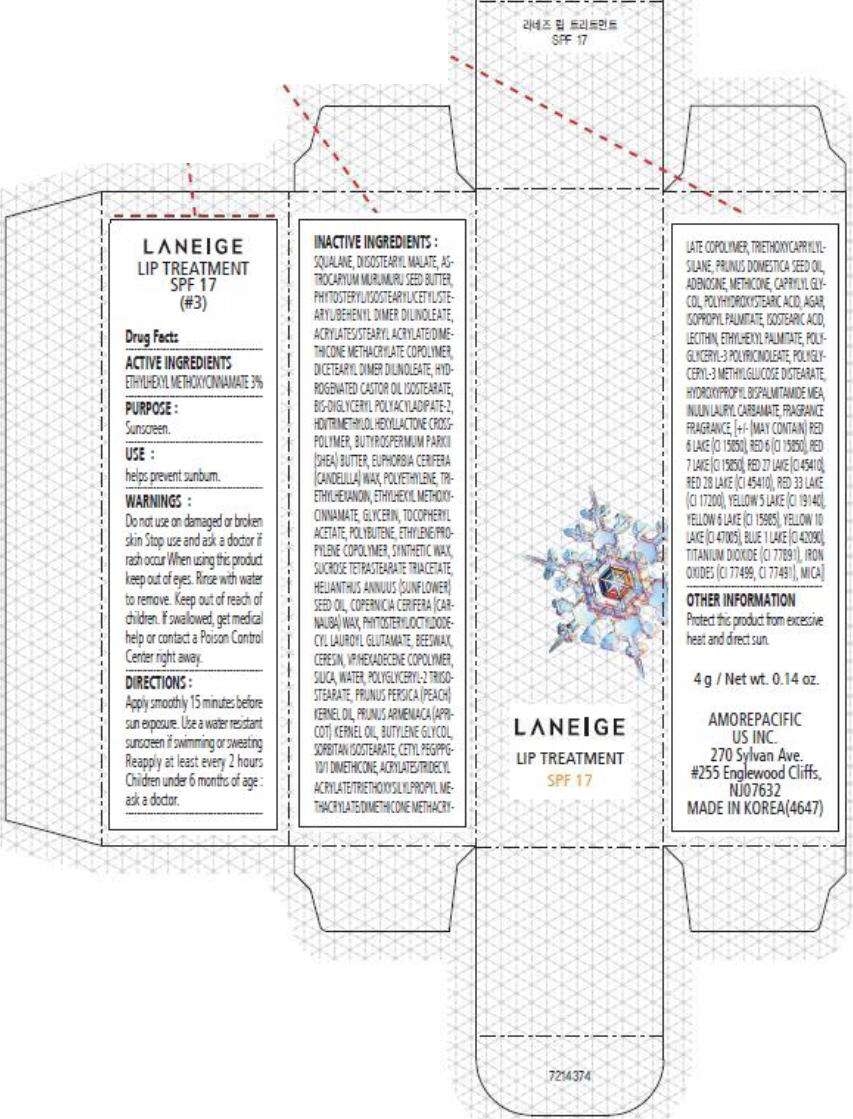
- PRINCIPAL DISPLAY PANEL - 4 g Carton (#3)
FULL PRESCRIBING INFORMATION
Drug Facts
ACTIVE INGREDIENTS
ETHYLHEXYL METHOXYCINNAMATE 3%
PURPOSE
Sunscreen.
USE
helps prevent sunburn.
WARNINGS
Do not use on damaged or broken skin
Stop use and ask a doctor if rash occur
When using this product keep out of eyes. Rinse with water to remove.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
Apply smoothly 15 minutes before sun exposure. Use a water resistant sunscreen if swimming or sweating Reapply at least every 2 hours Children under 6 months of age : ask a doctor.
INACTIVE INGREDIENTS
SQUALANE, DIISOSTEARYL MALATE, PHYTOSTERYL/ISOSTEARYL/CETYL/STEARYL/BEHENYL DIMER DILINOLEATE, ASTROCARYUM MURUMURU SEED BUTTER, ACRYLATES/STEARYL ACRYLATE/DIMETHICONE METHACRYLATE COPOLYMER, DICETEARYL DIMER DILINOLEATE, HYDROGENATED CASTOR OIL ISOSTEARATE, BIS-DIGLYCERYL POLYACYLADIPATE-2, HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, BUTYROSPERMUM PARKII (SHEA) BUTTER, EUPHORBIA CERIFERA (CANDELILLA) WAX, POLYETHYLENE, TRIETHYLHEXANOIN, ETHYLHEXYL METHOXYCINNAMATE, GLYCERIN, POLYBUTENE, TOCOPHERYL ACETATE, SYNTHETIC WAX, ETHYLENE/PROPYLENE COPOLYMER, SUCROSE TETRASTEARATE TRIACETATE, HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL, COPERNICIA CERIFERA (CARNAUBA) WAX, PHYTOSTERYL/OCTYLDODECYL LAUROYL GLUTAMATE, BEESWAX, POLYGLYCERYL-2 TRIISOSTEARATE, VP/HEXADECENE COPOLYMER, CERESIN, SILICA, WATER, PRUNUS ARMENIACA (APRICOT) KERNEL OIL, PRUNUS PERSICA (PEACH) KERNEL OIL, BUTYLENE GLYCOL, SORBITAN ISOSTEARATE, CETYL PEG/PPG-10/1 DIMETHICONE, PRUNUS DOMESTICA SEED OIL, ACRYLATES/TRIDECYL ACRYLATE/TRIETHOXYSILYLPROPYL METHACRYLATE/DIMETHICONE METHACRYLATE COPOLYMER, TRIETHOXYCAPRYLYLSILANE, ADENOSINE, CAPRYLYL GLYCOL, METHICONE, AGAR, POLYHYDROXYSTEARIC ACID, ISOPROPYL PALMITATE, ISOSTEARIC ACID, LECITHIN, ETHYLHEXYL PALMITATE, POLYGLYCERYL-3 POLYRICINOLEATE, POLYGLYCERYL-3 METHYLGLUCOSE DISTEARATE, INULIN LAURYL CARBAMATE, HYDROXYPROPYL BISPALMITAMIDE MEA, FRAGRANCE [+/- MAY CONTAIN) RED 6 LAKE (CI 15850), RED 6 (CI 15850), RED 7 LAKE (CI 15850), RED 27 LAKE (CI 45410), RED 28 LAKE (CI 45410), RED 33 LAKE (CI 17200), YELLOW 5 LAKE (CI 19140), YELLOW 6 LAKE (CI 15985), YELLOW 10 LAKE (CI 47005), BLUE 1 LAKE (CI 42090), TITANIUM DIOXIDE (CI 77891), IRON OXIDES (CI 77499, CI 77491), MICA]
OTHER INFORMATION
Protect this product from excessive heat and direct sun.
PRINCIPAL DISPLAY PANEL - 4 g Carton (#1)
LANEIGE
LIP TREATMENT
SPF 17
PRINCIPAL DISPLAY PANEL - 4 g Carton (#2)
LANEIGE
LIP TREATMENT
SPF 17
PRINCIPAL DISPLAY PANEL - 4 g Carton (#3)
LANEIGE
LIP TREATMENT
SPF 17
LANEIGE LIP TREATMENTOCTINOXATE STICK
| |||||||||||||||||||||||||||||||||||||||||||||||||
LANEIGE LIP TREATMENTOCTINOXATE STICK
| |||||||||||||||||||||||||||||||||||||||||||||||||
LANEIGE LIP TREATMENTOCTINOXATE STICK
| |||||||||||||||||||||||||||||||||||||||||||||||||