Labetalol HCl
Cantrell Drug Company
Labetalol HCl 5 mg/mL 4 mL Syringe
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Label
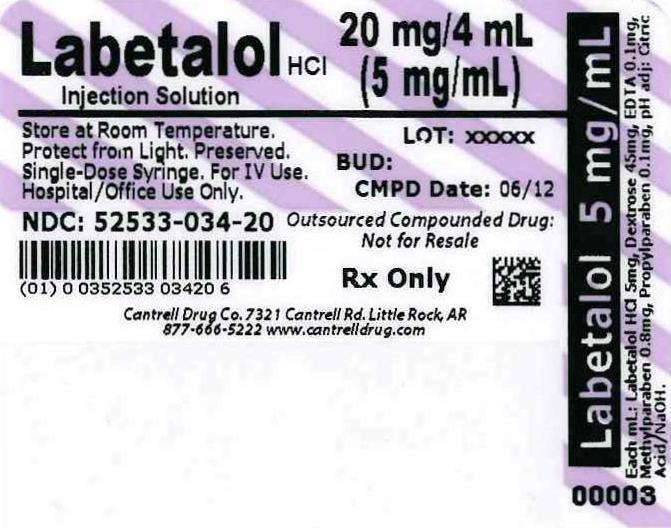
Labetalol HCl
Labetalol HCl INJECTION, SOLUTION
Product Information
|
|
Product Type
|
Human prescription drug label |
Item Code (Source)
|
NDC:52533-034 |
|
Route of Administration
|
INTRAVENOUS |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:52533-034-20 |
4 in 1 SYRINGE, PLASTIC |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
|
|
2012-02-23 |
|
|
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!
